




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����� | B������ | C������ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������ʵ���һ����� |
| B���������ṩ��H����������ṩ��OH�������ʵ������ |
| C��������������� |
| D����Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
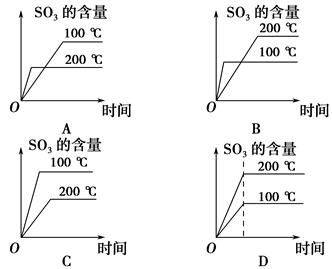
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ӳˮ�е�Ca2����Mg2�������������Ҫ�ɷֽ�϶����ɳ������Ӷ��˷ѷ��� |
| B��������Ca2����Mg2�����ӵ�ˮ����Ӳˮ |
| C��ˮ��Ӳ������CaO����Ϊ���� |
| D��1 Lˮ�к�10 mg CaO�����൱��10 mg CaO����Ϊ1�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
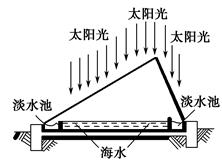
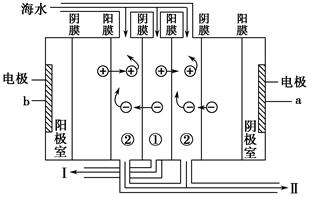
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ѧ������������ȼ�����������ѧ˵ʹ������ѧȡ���˸����Խ�չ |
| B��Ӣ����ѧ�����������״κϳ��˾���������Եĵ�����-------ţ�ȵ��� |
| C����̼���仯����Ϊԭ���Ƴɵ�оƬ���ά�������ǽ�������Ϣʱ�� |
| D��������ѧ���ǵIJ���Ŭ��������Ȼ���з����Լ��˹�������Ļ������Ѿ�����3500���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
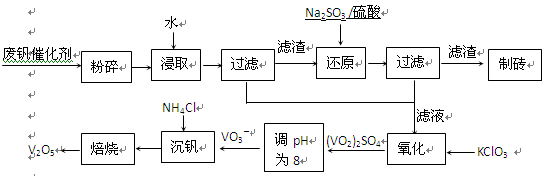
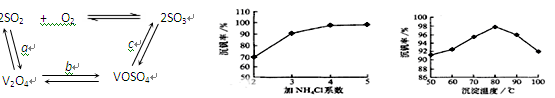
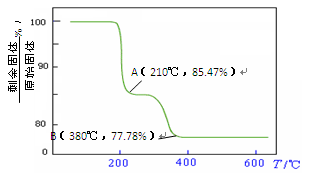
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com