
ЃЈ16ЗжЃЉФГЭЌбЇгУЯТЭМзАжУзіХЈСђЫсгыФОЬПЗДгІЕФЪЕбщЃЌвдМАМьбщЩњГЩЕФCO2КЭSO2ЁЃвбжЊЃКCжаЕФЫсадИпУЬЫсМиШмвКОпгаЧПбѕЛЏадЃЌФмЮќЪеОпгаЛЙдадЕФSO2ЦјЬхЁЃОнДЫЛиД№ЯТСаЮЪЬтЃК
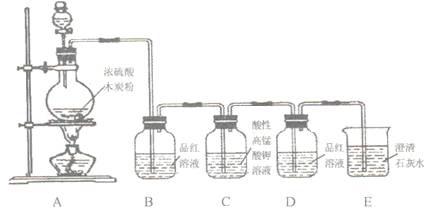
ЃЈ1ЃЉФОЬПгыХЈH2SO4дкМгШШЬѕМўЯТЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК
ЃЈ2ЃЉзАжУBЙлВьЕНЕФЯжЯѓЪЧ ЃЌЕУГіЕФНсТлЪЧ
ЃЈ3ЃЉзАжУDЙлВьЕНЕФЯжЯѓЪЧ ЃЌЕУГіЕФНсТлЪЧ
ЃЈ4ЃЉзАжУEЙлВьЕНЕФЯжЯѓЪЧ ЃЌЕУГіЕФНсТлЪЧ ЁЃ
ИУзАжУжаЗЂЩњЕФРызгЗНГЬЪНЪЧ ЁЃ
ЃЈ1ЃЉC+2H2SO4ЃЈХЈЃЉ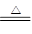 CO2Ёќ+2SO2ЁќЃЋ2H2O
CO2Ёќ+2SO2ЁќЃЋ2H2O
ЃЈ2ЃЉЦЗКьЭЪЩЋЃЛВњЮяжагаSO2ЃЛ ЃЈ3ЃЉЦЗКьВЛЭЪЩЋЃЛSO2вбГ§ОЁ
ЃЈ4ЃЉГЮЧхЪЏЛвЫЎБфЛызЧЃЛВњЮяжагаCO2ЃЛCO2+Ca2++2OH-=CaCaO3Ё§+H2O
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКЃЈ1ЃЉХЈСђЫсОпгаЧПбѕЛЏадЃЌдкМгШШЕФЬѕМўЯТЃЌФмАбФОЬПбѕЛЏЩњГЩCO2ЁЂSO2КЭЫЎЃЌЗДгІЕФЗНГЬЪНЪЧC+2H2SO4ЃЈХЈЃЉ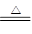 CO2Ёќ+2SO2ЁќЃЋ2H2OЁЃ
CO2Ёќ+2SO2ЁќЃЋ2H2OЁЃ
ЃЈ2ЃЉSO2ОпгаЦЏАзадЃЌФмЪЙЦЗКьШмвКЭЪЩЋЃЌЫљвдBжаЕФЪЕбщЯжЯѓЪЧЦЗКьЭЪЩЋЃЌетОЭЫЕУїЗДгІжагаSO2ЩњГЩЁЃ
ЃЈ3ЃЉгЩгкSO2ЛЙОпгаЛЙдадЃЌФмБЛЫсадИпУЬЫсМиШмвКбѕЛЏЃЌЫљвдCЕФзїгУЪЧГ§ШЅSO2ЃЌдђDжаЕФЦЗКьШмвКВЛдйЭЪЩЋЃЌетЫЕУїSO2вбОБЛЭъШЋГ§ОЁЁЃ
ЃЈ4ЃЉCO2ФмЪЙГЮЧхЕФЪЏЛвЫЎБфЛьзЧЃЌОнДЫПЩвдМьбщCO2ЕФДцдкЃЌЗДгІЕФРызгЗНГЬЪНЪЧCO2+Ca2++2OH-=CaCaO3Ё§+H2OЁЃ
ПМЕуЃКПМВщХЈСђЫсЕФаджЪЁЂЦјЬхЕФМьбщЕШ
ЕуЦРЃКгЩгкSO2вВФмЪЙГЮЧхЕФЪЏЛвЫЎБфЛьзЧЃЌЫљвддкМьбщCO2жЎЧАБиаыГ§ШЅSO2ЁЃЮЊБЃжЄSO2ЭъШЋБЛГ§ОЁЃЌЛЙашвЊдйЭЈЙ§ЦЗКьШмвКНјааМьбщЃЌетЪЧЩшМЦИУЪЕбщЕФЙиМќЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт

| ||
| ||
| ЕквЛДЮBжаШмвККЌгаРызг | ЕкЖўДЮBжаШмвККЌгаРызг | |
| Мз | гаFe2+ЃЌЮоFe3+ | гаSO 42- |
| вв | МШгаFe3+ЃЌгжгаFe2+ | гаSO 42- |
| Бћ | гаFe3+ЃЌЮоFe2+ | гаFe2+ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт

| ЪЕбщ ађКХ |
ЪЕбщВйзї | ЯжЯѓ | НсТл |
| Ђё | НЋТШЫЎЕЮШыЦЗКьШмвК | ЦЗКьШмвКЭЪЩЋ | ТШЦјгыЫЎЗДгІЕФВњЮягаЦЏАзад |
| Ђђ | ТШЫЎжаМгШыЬМЫсЧтФЦЗлФЉ | гаЮоЩЋЦј ХнВњЩњ |
ТШЦјгыЫЎЗДгІЕФВњЮяОпгаЫсад |
| ||
| ||
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2012-2013бЇФъИЃНЈНњНбје§жабЇИпвЛЩЯбЇЦкЦкжаПМЪдЮФПЦЛЏбЇЪдОэЃЈДјНтЮіЃЉ ЬтаЭЃКЪЕбщЬт
ЃЈ16ЗжЃЉФГЭЌбЇгУЯТЭМзАжУзіХЈСђЫсгыФОЬПЗДгІЕФЪЕбщЃЌвдМАМьбщЩњГЩЕФCO2КЭSO2ЁЃвбжЊЃКCжаЕФЫсадИпУЬЫсМиШмвКОпгаЧПбѕЛЏадЃЌФмЮќЪеОпгаЛЙдадЕФSO2ЦјЬхЁЃОнДЫЛиД№ЯТСаЮЪЬтЃК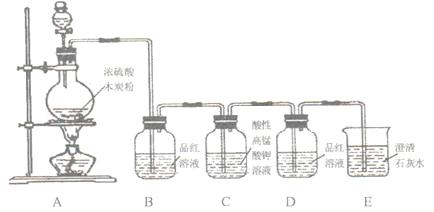
ЃЈ1ЃЉФОЬПгыХЈH2SO4дкМгШШЬѕМўЯТЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК
ЃЈ2ЃЉзАжУBЙлВьЕНЕФЯжЯѓЪЧ ЃЌЕУГіЕФНсТлЪЧ
ЃЈ3ЃЉзАжУDЙлВьЕНЕФЯжЯѓЪЧ ЃЌЕУГіЕФНсТлЪЧ
ЃЈ4ЃЉзАжУEЙлВьЕНЕФЯжЯѓЪЧ ЃЌЕУГіЕФНсТлЪЧ ЁЃ
ИУзАжУжаЗЂЩњЕФРызгЗНГЬЪНЪЧ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2011-2012бЇФъеуНЪЁКМжнЕиЧјЦпаЃИпвЛЯТбЇЦкЦкжаСЊПМЛЏбЇЪдОэЃЈДјНтЮіЃЉ ЬтаЭЃКЪЕбщЬт
ЃЈ13ЗжЃЉФГбаОПадбЇЯАаЁзщЩшМЦВЛЭЌЪЕбщЗНАИРДбаОПЯѕЫсЕФаджЪЁЃ
ЃЈ1ЃЉМззщЭЌбЇЩшМЦЯТЭМзАжУРДжЄЪЕЯЁЯѕЫсгыЭЗДгІЃЌВНжшШчЯТЁЃ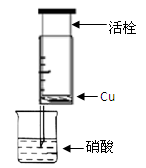
AЁЂМьбщ50mlеыЭВЕФЦјУмадЁЃ
BЁЂГщГіЛюЫЈЃЌЭљеыЭВФкЗХШывЛаЁПщЭЦЌЃЌАбеыЭВЛюЫЈЭЦЕНЕзЃЌНЋеыЭВЯТЖЫВЃСЇЙмНўШыеєСѓЫЎжаЃЌГщРЛюЫЈЃЌЮќШыдМ10mLеєСѓЫЎЃЌЗЂЯжеыЭВФкШдгаПеЦјЃЌШЛКѓ ЁЃ
CЁЂНЋеыЭВЯТЖЫВЃСЇЙмНўШыХЈЯѕЫсжаЃЌГщРЛюЫЈЃЌЛКЛКЮќШы4mLХЈЯѕЫсЃЌНЋеыЭВЯТЖЫЬзЩЯЯ№ЦЄЙмЃЌШЛКѓгУЬњМаМазЁЁЃ
ЛиД№вдЯТЯрЙиЮЪЬтЃК
ЂйBжагІВЙГфЕФВйзїЪЧ ЁЃ
ЂквЛЖЮЪБМфКѓдкеыЭВФкЙлВьЕНШмвКбеЩЋЮЊРЖЩЋЃЌга ЩЋЦјЬхВњЩњЁЃИУЗДгІЕФРызгЗНГЬЪНЮЊ ЁЃ
ЂлЗДгІЭЃжЙКѓЃЌеыЭВФкВњЩњСЫЦјЬхЃЌвЊбщжЄВњЩњЕФЦјЬхЪЧNOЃЌЛЙашНјвЛВННјааЕФВйзїЪЧ ЁЃ
ЃЈ2ЃЉввзщЭЌбЇвВгУДЫзАжУжБНгГщШЁХЈЯѕЫсКЭЭЗДгІЃЌЗЂЯжШмвКЪЧТЬЩЋЕФЃЌЮЊСЫЬНОПТЬЩЋЕФдвђЃЌввзщЭЌбЇгжзіСЫШ§зщЖдБШЪЕбщЃЌОпЬхШчЯТЃК
| зщ | mЃЈCuЃЉ/g | ЯѕЫсЃЈЙ§СПЃЉ | ШмвКбеЩЋ |
| A | 1 | ХЈHNO3(4mL) | ШмвКЮЊТЬЩЋ |
| 1 | ЯЁHNO3(4mL) | ШмвКЮЊРЖЩЋ | |
| B | 0.5 | ХЈHNO3(4mL) | ШмвКЮЊТЬЩЋ |
| 1 | ЯЁHNO3(4mL) | ШмвКЮЊРЖЩЋ | |
| C | 2 | ХЈHNO3(4mL) | ШмвКЮЊТЬЩЋ |
| 1 | ЯЁHNO3(4mL) | ШмвКЮЊРЖЩЋ |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com