
| �ζ� ���� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
���� ��1���ٵζ�����ҪҪNaOH��Һ��ϴ������ᵼ����ҺŨ��ƫ�ͣ����ƫ��
����ƿ���Ƿ���ˮ����ʵ������Ӱ�죬�ɴ����ʵ����ʵ����ĽǶȷ�����
�۵ζ�ʱ�۾�Ӧע��ע��۲���ɫ�仯����ȷ���յ㣻
�ܸ��ݷ�̪�ı�ɫ��Χȷ���ζ��յ�ʱ��ɫ�仯��
��2������Һ�����Ӧȡ����ʵ���ƽ��ֵ���������Һ��H+�����ʵ��������ݷ���ʽ��֪��CH2��6N4H+�����ʵ���������ȷ����Ʒ�е�������������
��� �⣺��1���ټ�ʽ�ζ���������ˮϴ�Ӻ���Ҫ����NaOH��Һ��ϴ�������൱��NaOH��Һ��ϡ�ͣ��ζ����ĵ������ƫ�ߣ������Ʒ�е�����������Ҳ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
����ƿ������ˮϴ�Ӻ���Ȼˮδ������������Һ�е�H+�����ʵ������䣬��ζ�ʱ����NaOH����Һ�е��������Ƶ����ʵ����Ͳ��䣬Ҳ������Ӱ�죬
�ʴ�Ϊ����Ӱ�죻
�۶�ʱ�ߵα�ҡ����ƿ���۾�Ӧע��۲���ɫ�仯��ȷ���ζ��յ㣬�ʴ�Ϊ��B��
�ܴ���ҺΪ���ԣ���̪ӦΪ��ɫ������ҺתΪ����ʱ����Һ��ɫ��Ϊ�ۺ죨��dz�죩�����һ��NaOH��Һ���£���Һ����ɫ���ۺ죨��dz�죩���Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����һ��NaOH��Һ���£���Һ����ɫ���ۺ죨��dz�죩���Ұ�����ڲ���ɫ��
��2������Һ�����Ӧȡ����ʵ���ƽ��ֵ��
����ȷ���ζ�ʱ���õ�NaOH����ҺΪ$\frac{20.01+19.99+20.00}{3}$mL=20.00mL��
�����������Լ�ȩ��Һһ���ǹ����ģ�����1.500g ��� ���ܽ��ȡ������$\frac{1}{10}$���еζ�����0.15g��
�ζ��������Һ�к���H+������CH2��6N4H+����0.02L����0.1010mol/L=0.00202mol��
����4NH4++6HCHO�T3H++6H2O+��CH2��6N4H+��ÿ����4molH+������CH2��6N4H+����������NH4+4mol��
���Թ�����NH4+0.00202mol��
���к���Ԫ��0.00202mol��14g/mol=0.02828g
���Ե�����������Ϊ$\frac{0.02828}{0.15}$��100%=18.85%��
�ʴ�Ϊ��18.85%��
���� ���⿼�����ʵĺ����IJⶨ���������к͵ζ��Ŀ��飬ע����ѧ��ʵ�������ͷ��������ͼ����������ۺϿ��飬Ϊ���Ը�Ƶ���㣬��Ŀ�ѶȲ���


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������������Ȼ�茶����� | B�� | ���ȷ�Ӧ | ||
| C�� | þ������ķ�Ӧ | D�� | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
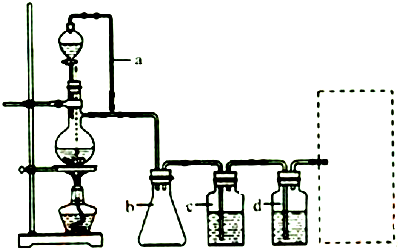
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 96% | B�� | 48% | C�� | 9.6% | D�� | 56% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��100�桢101 kPa�����£�Һ̬ˮ��������Ϊ40.69 kJ•mol-1����H2O��g��?H2O��l�� �ġ�H=+40.69 kJ•mol-1 | |||||||||||||||
| B�� | ��֪MgCO3��Ksp=6.82��10-6�������к��й���MgCO3����Һ�У�����c��Mg2+��=c��CO32-������c��Mg2+��•c��CO32-��=6.82��10-6 | |||||||||||||||
| C�� | ��֪ijЩ��ѧ���ļ����������±���
| |||||||||||||||
| D�� | �����£���0.10 mol•L-1��NH3•H2O��Һ�м�������NH4Cl���壬��ʹNH3•H2O�ĵ���Ƚ��ͣ���Һ��pH��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼������������θ�����˵�θ�����֢ | |
| B�� | �����ƾ���ǿ�Ļ�ԭ�ԣ��������ƺ�TiCl4��Һ��Ӧ��ȡ����Ti | |
| C�� | ���ࡢ��֬����������һ�������¾�����ˮ�� | |
| D�� | ������ˮ��Һ�ʼ��ԣ����������ƹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ${\;}_{17}^{37}$Cl2��Ħ��������74 | |
| B�� | ${\;}_{17}^{37}$Cl��${\;}_{17}^{35}$Cl��Ϊͬλ�أ�${\;}_{17}^{35}$Cl2��Cl2��Ϊͬ���칹�� | |
| C�� | ͨ������£���������������������Ҳ���л�ԭ�� | |
| D�� | ��ʹʪ��ĵ���KI��ֽ����ɫ������һ����Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ��
��Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ�� ��1mol O22+�к��еĦм���ĿΪ2NA����
��1mol O22+�к��еĦм���ĿΪ2NA�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com