
��10����1���ڱ�״���£�CO��CO2������������Ϊ36g�����Ϊ22.4L��
��CO��ռ������� L������Ϊ g�����������CO2�ķ�����Ϊ ��
��2��3.01��1023��OH�������ʵ���Ϊ mol������Ϊ g����ЩOH�������ʵ������״�������Ϊ L��NH3�����ʵ�����ȣ��� g Na�����е���������ͬ��
��3�����Ϊ0.5L ��1mol/L��CaCl2 ��Һ��Cl�������ʵ���Ũ��Ϊ___________mol/L
��4����״���£�33.6L��NH3�����е����ʵ���Ϊ_________mol�������ܽ���ˮ���1L����Һ������Һ�����ʵ���Ũ��Ϊ__________mol/L
��1�� 11.2 14 0.5NA ��2�� 0.5 8.5 11.2 11.5
��3�� 2 ��4�� 1.5 1.5
��������
�����������1���ڱ�״���£����Ϊ22.4L����֪Ϊ1mol������Ϊ36g�����������ƽ����Է�������Ϊ36����ʮ�ֽ��淨��֪CO��CO2�������Ϊ��36-28������44-36��=1��1 ����CO��ռ�������11.2L������Ϊ0.5mol��28g/mol=14g�����������CO2�ķ�����Ϊ0.5NA ����2����2��3.01��1023��OH�������ʵ���Ϊ3.01��1023/6.02��1023mol-1=0.5mol������Ϊ0.5mol��17g/mol=8.5g��0.5mol NH3�ڱ�״�������Ϊ0.5mol��22.4L/mol=11.2L��0.5molNa����������0.5mol��23g/mol=11.5g ����3����Һ�����ӵ�Ũ������Һ�����û�й�ϵ������Һ��Ũ�ȼ����ӵĸ����йأ�1mol/L��CaCl2 ��Һ��Cl�������ʵ���Ũ��Ϊ2mol/L����4����4����״���£�33.6L��NH3�����е����ʵ���Ϊ33.6L/22.4L/mol=1.5mol�������ܽ���ˮ���1L����Һ������Һ�����ʵ���Ũ��Ϊ1.5mol/1L=1.5mol/L ��
���㣺����Ħ����������ʵ���Ũ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ���㽭ʡ�����и�һ11���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ж������йػ�ѧ���������������ȷ����
A����Һ�뽺�壺��ͬ�ı���ԭ�����ܷ��������ЧӦ
B��������������Ƿ������һ��Ԫ��
C��������ԭ��Ӧ��Ԫ�ػ��ϼ��Ƿ�仯
D���������ǵ���ʣ����ʱ����ĵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ɹŰ��и�һ10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�鷽������У����е���
A����ϡ�������ˣ���ȥ����ͭ���е�����þ�ۺ�����
B������ȡ�ķ����������ͺ�ú��
C�����ܽ⡢���˵ķ�����������غ��Ȼ��ƹ���Ļ����
D���������������Ļ������ͨ�����ȵ�����ͭ���Գ�ȥ���е�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ�߶���ѧ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʽΪC5H7Br ���л����������
A��ֻ����1��˫����ֱ���л��� B������2��˫����ֱ���л���
C������1��˫���Ļ�״�л��� D������1��������ֱ���л���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ�߶���ѧ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�м�����������������̼������������ͬ��������˵����ȷ����
A������һ����ͬ���칹��
B�����Ҳ�������ͬϵ��
C�����ҵķ����У�̼����ԭ�Ӹ���֮����ͬ
D�����Ҹ�1 mol��ȫȼ�պ����ɵĶ�����̼������һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���з�Ӧ�����ӷ���ʽ��д��ȷ����
A���Ȼ�ͭ��Һ�����۷�Ӧ��Cu2++Fe=Fe2++Cu
B��ϡ H2SO4�����۷�Ӧ��2Fe+6H+=2Fe3++3H2��
C������������Һ��ϡ H2SO4 ��Ӧ��Ba2++SO42��=BaSO4��
D��̼��������ᷴӦ��CO32��+2H+=H2O+CO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ڱ�״���£����2.8L��������n����ԭ�ӣ����ӵ������ɱ�ʾΪ
A��n/8mol-1 B��n/16 mol-1 C��8n mol-1 D��4n mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и����еķ�Ӧ������ͬһ��Ӧ���͵���
A���������ˮ���Ʊ������ɱ�ϩ��ˮ��Ӧ�Ʊ���
B���ɼױ������ƶ������ױ����ɼױ������Ʊ�����
C�����ȴ���������ȥ�ƻ���ϩ���ɱ�ϩ������1,2?�������
D����������Ҵ��������������ɱ���������ˮ���Ʊ�������Ҵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ��̨�и߶�12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ý�屨����������ȼ�ϵ��Ϊ�����Ĺ����������ڱ��������С����ӽ���Ĥȼ�ϵ��(PEMFC)����Ϊ�綯�����Ķ���Դ����ȼ�ϵ��������Ϊȼ�ϣ�����Ϊ����������Ϊ�缫������������H�������жԸ�ȼ�ϵ�ص�������ȷ����
��������ӦΪ��O2��4H����4e��= 2H2O
�ڵ����ɸ�����������
���ܵĻ�ѧ��ӦΪ��2H2��O2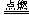 2H2O
2H2O
��������ͨ��������������ƶ�
A���٢ڢ� B���ڢۢ� C���٢ڢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com