
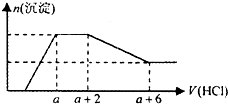
 ij��Һ�п��ܺ���OH-��CO32-��AlO2-��SiO32-��SO42-��HCO3-��Na+��Fe3+��Mg2+��Al3+�����ӣ��������Һ����μ���һ�����ʵ���Ũ�ȵ�������Һʱ���������ɳ��������ʵ�����������Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
ij��Һ�п��ܺ���OH-��CO32-��AlO2-��SiO32-��SO42-��HCO3-��Na+��Fe3+��Mg2+��Al3+�����ӣ��������Һ����μ���һ�����ʵ���Ũ�ȵ�������Һʱ���������ɳ��������ʵ�����������Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ԭ��Һ��һ�����е��������ǣ�OH-��SiO32-��AlO2-��CO32- | |
| B�� | ��Ӧ����γɵ���Һ�е�����ֻ��NaCl | |
| C�� | ԭ��Һ��һ������Na2SO4 ��NaOH | |
| D�� | ԭ��Һ�к���CO32-��AlO2-�����ʵ���֮��Ϊ1��1 |
���� �ṹͼ�����߱仯��֪����ʼ��������˵��������������Һ�еļӦ��˵����Һ��һ�������������ӣ��������������Ӳ��ܹ��������ΪFe3+��Mg2+��Al3+�����Ӧ���ɳ���������˵����AlO2-�������ӷ�Ӧ�����������������������������AlO2-��HCO3-������Ӧ����������������������Һ��һ��������HCO3-����������������������䣬�������������ֻ����CO32-���ӣ���Ӧ�������������ᣬ������С�������ٸı䣬��һ��֤��������������������������������������������ᣬ���ʣ�����Ϊ�����������Ӳ���ȷ�����ڣ���������Һ�ĵ����Կ�֪����Һ��һ���������������ӣ��Դ˽����⣮
��� �⣺��ͼ�������֪����ʼ��������˵��������������Һ�еļӦ��˵����Һ��һ����OH-���ӣ��������������Ӳ��ܹ��������ΪFe3+��Mg2+��Al3+�����Ӧ���ɳ���������˵����AlO2-��SiO32-�������ӷ�Ӧ�����������������������������AlO2-��HCO3-������Ӧ����������������������Һ��һ��������HCO3-����������������������䣬�������������ֻ����CO32-���ӣ���Ӧ�������������ᣬ������С�������ٸı䣬��һ��֤��������������������������������������������ᣬ���ʣ�����Ϊ�����������Ӳ���ȷ�����ڣ���������Һ�ĵ����Կ�֪����Һ��һ������Na+���ӣ�
A�������ж�ԭ��Һ��һ�����е��������ǣ�OH-��SiO32-��AlO2-��CO32-����A��ȷ��
B����Ӧ����γɵ���Һ�е�����ΪNaCl��AlCl3����B����
C����Һ����������Ӳ���ȷ����ʣ��ԭ��Һ��һ������Na2SO4����C����
D������ͼ���֪��̼������ӷ�Ӧ������Ϊ2�����CO32-+2H+=CO2��+H2O ���������ܽ����ĵ��������Ϊ4�����Al��OH��3+3H+=Al3++3H2O��ԭ��Һ�к���CO32-��AlO2-�����ʵ���֮��Ϊ3��4����D����
��ѡA��
���� ���⿼�������Ӽ���ķ���Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ����ͼ�����߱仯Ϊ����ؼ�����ȷ��������ӡ�ƫ��������ӡ����������Ļ�ѧ���ʣ�����������ѧ���ķ���������������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�� ��� | ʵ���¶� /�� | Na2S2O3 | H2SO4 | ����ˮ��� /mL | ||
| ���/mL | Ũ��/mol•L-1 | ���/mL | Ũ��/mol•L-1 | |||
| �� | 25 | 10 | 0.1 | 10 | 0.1 | 0 |
| �� | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
| �� | 25 | 5 | 0.2 | 10 | 0.2 | 5 |
| �� | 50 | 5 | 0.1 | 10 | 0.1 | 5 |
| �� | 50 | 10 | 0.2 | 5 | 0.2 | 5 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ñ���ȡ��ˮ�е���ʱ������ı���Һ�ӷ�Һ©���¿ڷų� | |
| B�� | �ò�����պȡ������ˮ������pH��ֽ�ϣ�Ȼ�����ɫ�����գ��ɲⶨ������ˮ��pHֵ | |
| C�� | ������������ͭ����Һ���Լ������ᡢ�����Ǻ͵���������Һ | |
| D�� | ��ǿ���ǿ�ʴ����ʱ��Ӧ���ô���ˮ��ϴ������2%������Һ��������Һϴ�������ˮ��ϴ�������������һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

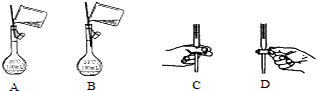
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������NDM-l���������п����ض����п�ҩ�ԣ������ʺܸߣ�Ϊ��ֹ���������ĸ�Ⱦ��Ҫ��ǿ���������˵�������������������������ѡ�ú�����������˫��ˮ���ƾ����� �˵����� | |
| B�� | ���������������ڽ��ػ����������������ָ��£������������ǽ������� | |
| C�� | ���л��������ܻ������������ö�����������ͨ�����˷�����ȥ | |
| D�� | ��ɫ��ѧ�ĺ�����Ӧ�û�ѧԭ���Ի�����Ⱦ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ҵ��F������MnO2��AF���Ʊ� | |
| B�� | BԪ�����γɵĵ��ʵľ������Ͷ�����ͬ�� | |
| C�� | F���γɵ��⻯�������ǿ��BD2��ˮ��������ԣ�˵��F�ķǽ�����ǿ��B | |
| D�� | �ɻ�ѧ���Ƕ��ƶϣ����γ�BDF2���ֻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| a | b | c | d | |
| �� | �������ɫ���� | �������ɫ���� | �������ɫ���� | �������ɫ���� |
| �� | ��ʯ�� | �轺 | ��ʯ�� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������KMnO4��Һ��Fe2+������Fe3+����ת��ΪFe��OH��3������ȥ | |
| B�� | ��ZnO���ڽ���Һ������ԣ���ʹijЩ�����γ������������ | |
| C�� | ��ʵ�����������У�����Ag2SO4�ɳ�ȥCl-���������˳���ת����ԭ�� | |
| D�� | Ҳ������ZnCO3����ZnO������Һ������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com