
ij��ѧ����С��Ϊ�ⶨ������CO2�ĺ���������������ʵ�飺
(1)����0.100 0 mol��L��1��0.010 00 mol��L��1�ı����ᡣ
(2)��0.100 0 mol��L��1�ı�����ζ�δ֪Ũ�ȵ�Ba(OH)2��Һ10.00 mL�������ȥ����19.60 mL��
(3)�òⶨ��Ba(OH)2��Һ���ն���������CO2��ȡBa(OH)2��Һ10.00 mL������100 mL����ƿ���ˮ���̶��ߡ�ȡ��ϡ�ͺ����Һ�����ܱ������ڣ���ͨ��10 L��״���µĿ���������ʱ���ɳ�����
(4)��������������Һ��
(5)ȡ��Һ20.00 mL����0.010 00 mol��L��1������ζ�����ȥ����34.80 mL����ش��������⣺
�����Ʊ�����ʱ������������Щ����________��
A��������ƽ��B������ƿ��C����ʽ�ζ��ܡ�D����Ͳ��E���ձ���F����ͷ�ιܡ�G��������
�ڵζ������У�����__________������__________���۾�________________________��
��Ba(OH)2��Һ�����ʵ���Ũ����________________________________________��
�ܹ���������Һ��Ŀ����_________________________________________________��
�ݴ˿�����Ʒ�к�CO2���������Ϊ______________________________________��
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һƿ�������Һ�����п��ܺ���H+��NH4+��Mg2+��Ba2+��Al3+��I-��NO3-��CO32-��SO42-��AlO2-��ȡ����Һ��������ʵ�飺
(1)ȡpH��ֽ���飬��Һ�����ԣ������ų�__________�Ĵ��ڡ�
(2)ȡ��������Һ����������CCl4������������ˮ������CCl4���Ϻ�ɫ�������ų�____ __�Ĵ��ڡ�
(3)��ȡ��������Һ��μ���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ���Ӧ�����о��������������ֿ��ų�________�Ĵ��ڡ�
(4)ȡ������(3)�еļ�����Һ����Na2CO3��Һ���а�ɫ�������ɣ�֤����________�Ĵ��ڣ������ų�_____�Ĵ��ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ��ȡ����ˮ��ʵ���У�û���漰���������� �� ��
A���ƾ��� B�������� C������̨ D���ձ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڿ��淴ӦA(g)��3B(s)
 2C(g)��2D(g)���ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ�ķ�Ӧ���������� (����)
2C(g)��2D(g)���ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ�ķ�Ӧ���������� (����)
A��v(A)��0.5 mol��L��1��min��1B��v(B)��1.2 mol��L��1��s��1
C��v(D)��0.4 mol��L��1��min��1D��v(C)��0.1 mol��L��1��s��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����ɱ���ܱ������У�����һ������X��Y��������ӦmX(g)
 nY(g)����H��Q kJ��mol��1����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ��
nY(g)����H��Q kJ��mol��1����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ��
|
�¶�/�� | 1 | 2 | 4 |
| 100 | 1.00 | 0.75 | 0.53 |
| 200 | 1.20 | 0.90 | 0.63 |
| 300 | 1.30 | 1.00 | 0.70 |
����˵����ȷ���� (����)
A��m>n
B��Q<0
C���¶Ȳ��䣬ѹǿ����Y��������������
D��������䣬�¶����ߣ�ƽ�����淴Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������մ�ϵ�к�������մ�ϵ�������������ǽ�������,���������������������,�������������ǵ�(����)
A.���º�ǿ�ȸ� B.��ѧ����
C.��ѧ���� D.���﹦��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ĸ�ת��,���в�����ͨ��һ����Ӧʵ�ֵ���(����)
��SiO2��Na2SiO3 ��CuSO4��CuCl2
��SiO2��H2SiO3 ��CuO��Cu(OH)2
A.�٢ڡ�����B.�ۢܡ�����C.�ڢۡ�����D.�ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڼ���ʱ��Ũ������ͭ������Ӧ�Ļ�ѧ����ʽΪ��
2H2SO4��Ũ����Cu  CuSO4��SO2����2H2O�����ڸ÷�Ӧ������˵���в���ȷ����
CuSO4��SO2����2H2O�����ڸ÷�Ӧ������˵���в���ȷ����
A����������ԭ��Ӧ B��ͭ�ǻ�ԭ��
C��H2SO4�����������Ժ����� D����Ӧ��ͭԪ�صĻ��ϼ۽���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
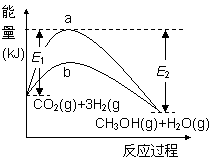
һ�������·�����Ӧ��
CO2(g)+3H2(g)  CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й����е������仯��
CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й����е������仯��
��ͼ������___(�a����b��)��ʾʹ�ô���ʱ�ķ�Ӧ���̡�ʹ�ô����Ը÷�Ӧ��Ӱ����______(��ѡ����ĸ)��
 A.��߷�Ӧ����
A.��߷�Ӧ����
B.���CO2��ת����
C.���ͷ�Ӧ���
D.�ı䷴Ӧ�Ħ�H
 ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)�Ļ�ѧƽ�ⳣ���ı���ʽK=_______________________�������¶ȣ�Kֵ��_______(���������С�����䡱)��
��CO2(g)+3H2(g) CH3OH(g)+H2O(g)�Ļ�ѧƽ�ⳣ���ı���ʽK=_______________________�������¶ȣ�Kֵ��_______(���������С�����䡱)��
���ú�E1��E2�ı���ʽ��ʾ
 CO2(g)+3H2(g) CH3OH(g)+H2O(g)�Ħ�H=______ kJ��mol��1
CO2(g)+3H2(g) CH3OH(g)+H2O(g)�Ħ�H=______ kJ��mol��1
��һ���¶��£������Ϊ2L���ݻ��̶����ܱ������У�����2molCO2��6molH2����10min��Ӧ�ﵽƽ��״̬W������1molCH3OH��CO2��ת����Ϊ________���ӷ�Ӧ��ʼ��ƽ�⣬��H2��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)=____________�����¶��£������Ϊ1L���ݻ��̶����ܱ������У����淴Ӧ��ʼ������ѧƽ�⣬�Ҹ���ֵ�ƽ��Ũ����ƽ��״̬W��ȫ��ͬ������ʼʱ����������n(CH3OH)=________��n(H2O)=________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com