
| | ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թܼ���__________ | ��������ų��ҳ���ʯ��ˮδ�����ǣ������________������ |
| �� | | |
| �� | | |
| | ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թܼ���___1mol/L��ϡ����____(1��) | ��������ų��ҳ���ʯ��ˮδ�����ǣ� �����___һ_____������(1��) |
| �� | ʵ�����ݼ����裺(1��) ȡ������Ʒ���Թ��У�����������ϡ���ᣬ����������������ͨ��Ʒ����Һ�ͳ���ʯ��ˮ | ����__Ʒ�첻��ɫ�� ��ʯ��ˮ�����_________(1��) ���ۣ�����__��___���� (1��) |
| �� | ʵ�����ݼ����裺(1��) ȡ������Ʒ���Թ��У�����������ϡ���ᣬ����������������ͨ��Ʒ����Һ�ͳ���ʯ��ˮ | ���� Ʒ����Һ��ɫ�� ����ʯ��ˮ�����____(1��)�� ���ۣ����������� |


 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������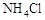 ����ʱ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ����� ����ʱ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����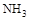 ������ ������ |
B����ij��Һ�м������������ữ���������ټ���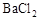 ��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�д��� ��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�д���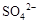 |
| C������к͵ζ�ʵ�����õ��IJ�������������ʽ�ζ��ܡ���ʽ�ζ��ܺ��ձ� |
D������Ͳ��ȡ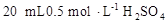 ��Һ���ձ��У���ˮ80mL,�����Ƴ�0.1 ��Һ���ձ��У���ˮ80mL,�����Ƴ�0.1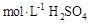 ��Һ ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
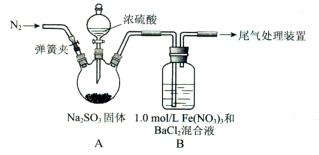
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ƿ�еļ������ϩ����ȥ����Ƭ���ֱ��ȼ���۲�������ɫ���Ƿ��к��� |
| B������ƾ��е�����ˮ����ƾ��м���������ʯ�� |
| C���Ʊ������飨C2H5Cl����������������Ļ�������ڹ��������·�Ӧ |
| D����ȥ��Ȳ���������壺��ʵ�����Ƶõ���Ȳ����ͨ������ͭ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
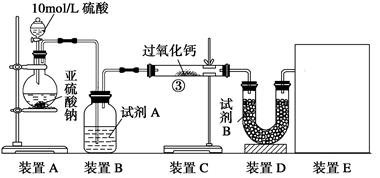
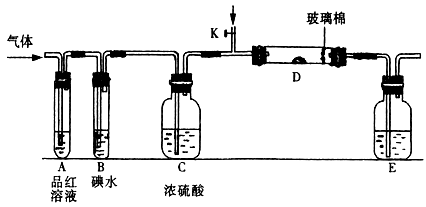
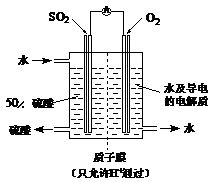
 ��֬Ư����������������������Ϊ�������������SO2ͨ��������ƹ����ĩ��������
��֬Ư����������������������Ϊ�������������SO2ͨ��������ƹ����ĩ�������� ���ɡ����������CO2��SO2�������
���ɡ����������CO2��SO2������� �Ƶķ�Ӧԭ����ͬ����Ҳ���������SO2���н�ǿ�Ļ�ԭ�ԣ�CO2��ǿ��ԭ�ԣ���Ӧԭ������ͬ���ݴ��������ʵ����������ж�
�Ƶķ�Ӧԭ����ͬ����Ҳ���������SO2���н�ǿ�Ļ�ԭ�ԣ�CO2��ǿ��ԭ�ԣ���Ӧԭ������ͬ���ݴ��������ʵ����������ж� ��
��
 �����
����� �����ڿ��л���װ��ͼ��
�����ڿ��л���װ��ͼ�� ��C����m1 g��װ��D����m2 g��װ��E���ռ���V L����(�ѻ���ɱ�״����)���������йز��������жϣ���SO2δ����ʱ��V��m1��m2�Ĺ�ϵʽΪ_________��
��C����m1 g��װ��D����m2 g��װ��E���ռ���V L����(�ѻ���ɱ�״����)���������йز��������жϣ���SO2δ����ʱ��V��m1��m2�Ĺ�ϵʽΪ_________�� ��________________________________________________________________��
��________________________________________________________________�� �����ۣ�
�����ۣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
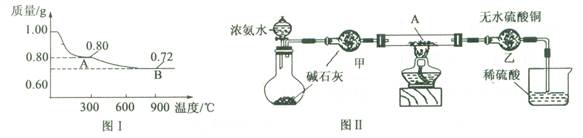
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
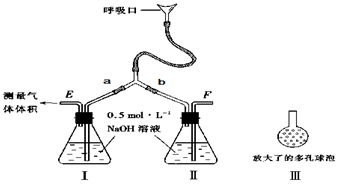
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

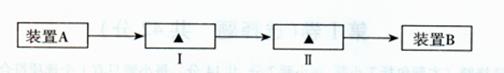
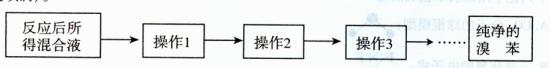
 ������2Ŀ�ģ� ��
������2Ŀ�ģ� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com