
| ������ | K+ | Fe2+ | Ca2+ | Ba2+ |
| ������ | NO3- | CO3-2 | SiO32- | SO42- |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�м�������ϡ���� | ������ɫ��״�������ų���״����0.56L���� |
| �� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4g |
| �� | ������Һ�еμ�BaCl2��Һ | ���������� |
���� ������ҺΪ������Һ��֪����Һ�к��е�����һ���ܹ��ܴ������棻
��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ$\frac{\frac{0.56L}{22.4L/mol}}{0.1L}$=0.25mol/L����һ��û��Ba2+��Ca2+��
�����ɰ�ɫ��״�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����������ȷֽ����ɶ������裬��������Ϊ2.4gΪ������������������ݹ�ԭ���غ㣬SiO32-��Ũ��Ϊ$\frac{\frac{2.4g}{60g/mol}}{0.1L}$=0.4mol/L��
��ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ��ݴ˽��н��
��� �⣺������ҺΪ������Һ��֪����Һ�к��е�����һ���ܹ��ܴ������棻
��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ$\frac{\frac{0.56L}{22.4L/mol}}{0.1L}$=0.25mol/L����һ��û��Ag+��Mg2+��
�����ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����������ȷֽ����ɶ������裬��������Ϊ2.4gΪ������������������ݹ�ԭ���غ㣬SiO32-��Ũ��Ϊ$\frac{\frac{2.4g}{60g/mol}}{0.1L}$=0.4mol/L��
��ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
��1����ʵ����֪����������ϡ�������ɰ�ɫ�������ڱ�״���·ų�0.56L���壬�����Һ��һ������CO32-��SiO32-����һ��û��Ca2+��Ba2+��
�ʴ�Ϊ��Ca2+��Ba2+��
��2�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����ʵ�����������������ӷ���ʽΪ��CO32-+2H+=CO2��+H2O��
�ʴ�Ϊ��CO32-+2H+=CO2��+H2O
��3����ʵ����֪��һ����������������ӣ�
�ʴ�Ϊ��SO42-��
��4�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L��
�ʴ�Ϊ���������ӣ������Ӵ��ĵ���������������������ĵ��������0.8��
���� ���⿼�����ӹ��桢���Ӽ��鼰������ʵ���Ũ�ȵļ��㣬��Ŀ�Ѷ��еȣ�ע���������ӷ�Ӧ��Ӧ�������������ӵļ��鷽�������ݵ���غ��ж�K+�Ƿ���ڣ��DZ�����ѵ㡢�״��㣮


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ڢܢ� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10086 | B�� | 10010 | C�� | 95518 | D�� | 120 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��ɫ����Һ�У�Fe2+��Al3+��NO3-��Cl-��S2- | |
| B�� | �������ۺ����������������Һ�У�NH4+��Na+��NO3-��Cl- | |
| C�� | 0.1 mol•L-1��NaHCO3��Һ�У�K+��Na+��SO42-��CO32- | |
| D�� | ����Mg�ܷų�H2����Һ�У�Mg2+��NH4+��ClO-��K+��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 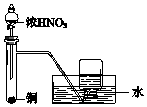 ��ȡ�������� | B�� |  ��ȡ���� | ||
| C�� | 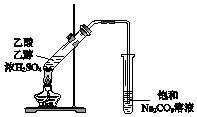 ���������� | D�� |  ����ϩ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ƿ�ʹ�����ʱ��ԣ���������ɱ�� | |
| B�� | ��Ȼ������������ǿ���ǿ����Һ�������Ի����� | |
| C�� | ʳ�Ρ��ǡ�������ζ������������ʳƷ������ | |
| D�� | �������װ��п�飬�������������������������з��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ö����ЧӦ��������Fe��OH��3�����FeCl3��Һ | |
| B�� | ����100g 20%���������Һ����Ҫ�õ�����ƿ | |
| C�� | ��������ȡ�ķ����������ˮ����ȡ���� | |
| D�� | ��ɫ��Ӧʵ��ǰ��Ӧ����ϡ����ϴ����˿�����ھƾ�������������ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij���ʽ�����ɫ��Ӧʱ���ֻ�ɫ�����������һ������Na+ | |
| B�� | �����Ȼ�����Һ�а�ɫ�����������ټ����ᣬ��������ʧ��һ����SO42- | |
| C�� | ij��Һ�м��������ܲ���ʹ����ʯ��ˮ����ǵ����壬����Һ�в�һ������CO32- | |
| D�� | ����̼������Һ������ɫ�������ټ������ɫ������ʧ��һ����Ba2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com