
£Ø8·Ö£©ÓŅĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż£¬øł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
| ŃĪĖį ·Ö×ÓŹ½£ŗHCl Ļą¶Ō·Ö×ÓÖŹĮæ£ŗ36.5 ĆÜ¶Č£ŗ1.19g/m3 ÖŹĮæ·ÖŹż£ŗ36.5% |
£Ø1£©øĆÅØŃĪĖįÖŠHClµÄĪļÖŹµÄĮæÅضČĪŖ mol/L”£
£Ø2£©Č”ÓĆČĪŅāĢå»żµÄøĆŃĪĖįČÜŅŗŹ±£¬ĻĀĮŠĪļĄķĮæ²»ĖęĖłČ”Ģå»ż¶ąÉŁ ¶ų±ä»ÆµÄŹĒ ”£
A”¢ČÜŅŗÖŠHClµÄÖŹĮæ B”¢ČÜŅŗµÄp H
C”¢ČÜŅŗÖŠH+µÄŹżÄæ D”¢ČÜŅŗµÄĆܶČ
£Ø3£©ÓūÅäÖĘÉĻŹöÅØŃĪĖį£¬ŠčŅŖŌŚ1LĖ®ÖŠĶØČė±źĢ¬ĻĀ L
HClĘųĢå£Ø±£Įō1Ī»Š”Źż£©
£Ø4£©ĻÖÓŠ1L1mol/LµÄĻ”ŃĪĖį£¬ÓūŹ¹ĘäÅضČŌö“ó1±¶£¬²ÉČ”µÄ“ėŹ©ŗĻĄķµÄŹĒ ”£
A”¢ĶØČė±źæöĻĀHClĘųĢå22.4L B”¢½«ČÜŅŗ¼ÓČČÅØĖõÖĮ0.5L
C”¢¼ÓČė5mol/LµÄŃĪĖį0.6L£¬ŌŁĻ”ŹĶÖĮ2L”£ D”¢¼ÓČė1L 3mol/LµÄŃĪĖį»ģŗĻ¾łŌČ”£
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
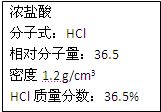 ÓŅĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©£¬ŹŌøł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
ÓŅĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©£¬ŹŌøł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÖŲĒģŹŠĪ÷ÄĻŹ¦“óø½ÖŠøßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
ÓŅĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©£¬ŹŌ øł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
øł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆÅØŃĪĖįÖŠHClµÄĪļÖŹµÄĮæÅضČĪŖ mol/L”£
£Ø2£©Č”ÓĆČĪŅāĢå»żµÄøĆŃĪĖįČÜŅŗŹ±£¬ĻĀĮŠĪļĄķĮæÖŠ²»ĖęĖłČ”Ģå»żµÄ¶ąÉŁ¶ų±ä»ÆµÄŹĒ ”£
A£®ČÜŅŗÖŠHClµÄĪļÖŹµÄĮæ B£®ČÜŅŗµÄÅضČ
C£®ČÜŅŗÖŠCl£µÄŹżÄæ  D£®ČÜŅŗµÄĆܶČ
D£®ČÜŅŗµÄĆܶČ
£Ø3£©Ä³Ń§ÉśÓūÓĆÉĻŹöÅØŃĪĖįŗĶÕōĮóĖ®ÅäÖĘ500 mLĪļÖŹµÄĮæÅضČĪŖ0.30 mol/LĻ”ŃĪĖį”£
¢ŁøĆѧɜŠčŅŖĮæČ” mLÉĻŹöÅØŃĪĖį½ųŠŠÅäÖĘ”£
¢ŚÅäÖĘŹ±£¬ĘäÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ(ÓĆ×ÖÄø±ķŹ¾£¬Ćæøö×ÖÄøÖ»ÄÜÓĆŅ»“Ī) ”£
A£®ÓĆ30 mLĖ®Ļ“µÓÉÕ±2”«3“Ī£¬Ļ“µÓŅŗ¾ł×¢ČėČŻĮæĘ棬ĒįĒįŅ”¶ÆČŻĮæĘæ
B£®ÓĆĮæĶ²×¼Č·ĮæČ”ĖłŠčÅØŃĪĖįµÄĢå»ż£¬ĀżĀżŃŲ±±Ś×¢ČėŹ¢ÓŠÉŁĮæĖ®£ØŌ¼30 mL£©µÄÉÕ±ÖŠ£¬ÓĆ²£Į§°ōĀżĀż½Į¶Æ£¬Ź¹Ęä»ģŗĻ¾łŌČ
C£®½«ŅŃĄäČ“µÄŃĪĖįŃŲ²£Į§°ō×¢Čė500 mLµÄČŻĮæĘæÖŠ
D£®½«ČŻĮæĘæµÄ²£Į§ČūøĒ½ō£¬µßµ¹Ņ”ŌČ
E£®øÄÓĆ½ŗĶ·µĪ¹Ü¼ÓĖ®£¬Ź¹ČÜŅŗ°¼ĆęĒ”ŗĆÓėæĢ¶ČĻßĻąĒŠ
F£®¼ĢŠųĶłČŻĮæĘæÄŚŠ”ŠÄ¼ÓĖ®£¬Ö±µ½ŅŗĆę½Ó½üæĢ¶ČĻß1”«2 cm“¦
¢ŪŌŚÅäÖĘ¹ż³ĢÖŠ£¬ĻĀĮŠ²Ł×÷»įŹ¹ĖłÅäÖʵÄĻ”ŃĪĖįĪļÖŹµÄĮæÅضČĘ«øߵďĒ ”£
A£®ÓĆĮæĶ²ĮæČ”ÅØŃĪĖįŹ±ø©ŹÓ¹Ū²ģ°¼ŅŗĆę
B£®ČÜŅŗ×¢ČėČŻĮæĘæĒ°Ć»ÓŠ»Öø“µ½ŹŅĪĀ¾Ķ½ųŠŠ¶ØČŻ
C£®¶ØČŻŹ±ŃöŹÓæĢ¶ČĻß
D£®ŌŚÅäÖĆĒ°ÓĆŅŃÖŖÅØ¶ČµÄĻ”ŃĪĖįČóĻ“ČŻĮæĘæ
£Ø4£©ĻÖ½«200 mL 0.30 mol/LµÄŃĪĖįÓė50 mL 0.80 mol/L CaCl2ČÜŅŗ»ģŗĻ(Ģå»ż±ä»ÆŗöĀŌ²»¼Ę)£¬ĖłµĆČÜŅŗÖŠCl£µÄĪļÖŹµÄĮæÅØ¶ČŹĒ mol/L£»ĻņĖłµĆ»ģŗĻČÜŅŗÖŠ¼ÓČė5.3 g Na2CO3¹ĢĢ壬³ä·Ö·“Ó¦ŗó£¬ČÜŅŗÖŠÉś³É³ĮµķµÄÖŹĮæÓŠ_________g”£
£Ø5£©ŌŚ±ź×¼×“æöĻĀ£¬½«______________L HClĘųĢåČÜÓŚ1000 mLĖ®ÖŠ(Ė®µÄĆܶČĪŖ1 g/cm3)£¬ĖłµĆŃĪĖįµÄĆܶČĪŖ1.2 g/cm3£¬ČÜÖŹÖŹĮæ·ÖŹżĪŖ36.5% ”£(±£ĮōŠ”ŹżµćŗóŅ»Ī»)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗÓÄĻŹ”10-11ѧğøßŅ»µŚŅ»“ĪŌĀ漣ػÆѧ£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©ÓŅĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż£¬øł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
|
ŃĪĖį ·Ö×ÓŹ½£ŗHCl Ļą¶Ō·Ö×ÓÖŹĮæ£ŗ36.5 ĆÜ¶Č£ŗ1.19g/m3 ÖŹĮæ·ÖŹż£ŗ36.5% |
£Ø1£©øĆÅØŃĪĖįÖŠHClµÄĪļÖŹµÄĮæÅضČĪŖ mol/L”£
£Ø2£©Č”ÓĆČĪŅāĢå»żµÄøĆŃĪĖįČÜŅŗŹ±£¬ĻĀĮŠĪļĄķĮæ²»ĖęĖłČ”Ģå»ż¶ąÉŁ ¶ų±ä»ÆµÄŹĒ ”£
A”¢ČÜŅŗÖŠHClµÄÖŹĮæ B”¢ČÜŅŗµÄp H
C”¢ČÜŅŗÖŠH+µÄŹżÄæ D”¢ČÜŅŗµÄĆܶČ
£Ø3£©ÓūÅäÖĘÉĻŹöÅØŃĪĖį£¬ŠčŅŖŌŚ1LĖ®ÖŠĶØČė±źĢ¬ĻĀ L
HClĘųĢå£Ø±£Įō1Ī»Š”Źż£©
£Ø4£©ĻÖÓŠ1L1mol/LµÄĻ”ŃĪĖį£¬ÓūŹ¹ĘäÅضČŌö“ó1±¶£¬²ÉČ”µÄ“ėŹ©ŗĻĄķµÄŹĒ ”£
A”¢ĶØČė±źæöĻĀHClĘųĢå22.4L B”¢½«ČÜŅŗ¼ÓČČÅØĖõÖĮ0.5L
C”¢¼ÓČė5mol/LµÄŃĪĖį0.6L£¬ŌŁĻ”ŹĶÖĮ2L”£ D”¢¼ÓČė1L 3mol/LµÄŃĪĖį»ģŗĻ¾łŌČ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģÖŲĒģŹŠøßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£¬ĆææÕ2·Ö£©ÓŅĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©£¬ŹŌøł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©Ä³Ń§ÉśÓūÓĆÉĻŹöÅØŃĪĖįŗĶÕōĮóĖ®ÅäÖĘ500 mLĪļÖŹµÄĮæÅضČĪŖ0.30 mol/LĻ”ŃĪĖį”£
¢ŁøĆѧɜŠčŅŖĮæČ” mLÉĻŹöÅØŃĪĖį½ųŠŠÅäÖĘ”£
¢ŚÅäÖĘŹ±£¬ĘäÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ(ÓĆ×ÖÄø±ķŹ¾£¬Ćæøö×ÖÄøÖ»ÄÜÓĆŅ»“Ī) ”£
A.ÓĆ30mLĖ®Ļ“µÓÉÕ±2”«3“Ī£¬Ļ“µÓŅŗ¾ł×¢ČėČŻĮæĘ棬ĒįĒįŅ”¶ÆČŻĮæĘæ
B.ÓĆĮæĶ²×¼Č·ĮæČ”ĖłŠčÅØŃĪĖįµÄĢå»ż£¬ĀżĀżŃŲ±±Ś×¢ČėŹ¢ÓŠÉŁĮæĖ®£ØŌ¼30 mL£©µÄÉÕ±ÖŠ£¬ÓĆ²£Į§°ōĀżĀż½Į¶Æ£¬Ź¹Ęä»ģŗĻ¾łŌČ
C.½«ŅŃĄäČ“µÄŃĪĖįŃŲ²£Į§°ō×¢Čė500mLµÄČŻĮæĘæÖŠ
D.½«ČŻĮæĘæµÄ²£Į§ČūøĒ½ō£¬µßµ¹Ņ”ŌČ
E.øÄÓĆ½ŗĶ·µĪ¹Ü¼ÓĖ®£¬Ź¹ČÜŅŗ°¼ĆęĒ”ŗĆÓėæĢ¶ČĻßĻąĒŠ
F.¼ĢŠųĶłČŻĮæĘæÄŚŠ”ŠÄ¼ÓĖ®£¬Ö±µ½ŅŗĆę½Ó½üæĢ¶ČĻß1”«2cm“¦
¢ŪŌŚÅäÖĘ¹ż³ĢÖŠ£¬ĻĀĮŠ²Ł×÷»įŹ¹ĖłÅäÖʵÄĻ”ŃĪĖįĪļÖŹµÄĮæÅضČĘ«øߵďĒ ”£
A.ÓĆĮæĶ²ĮæČ”ÅØŃĪĖįŹ±ø©ŹÓ¹Ū²ģ°¼ŅŗĆę
B.Ņ”ŌČŗó£¬ŅŗĆęĻĀ½µ£¬²¹³äĖ®
C.¶ØČŻŹ±ŃöŹÓæĢ¶ČĻß
D.ŌŚÅäÖĘĒ°ÓĆĻąĶ¬ÅØ¶ČµÄĻ”ŃĪĖįČóĻ“ČŻĮæĘæ
£Ø2£©ĻÖ½«200mL0.30mol/LµÄŃĪĖįÓė50mL0.80mol/LCaCl2ČÜŅŗ»ģŗĻ(»ģŗĻŗóĢå»ż±ä»ÆŗöĀŌ²»¼Ę)£¬ĖłµĆČÜŅŗÖŠCl£µÄĪļÖŹµÄĮæÅØ¶ČŹĒ mol/L”£
£Ø3£©ŌŚ±ź×¼×“æöĻĀ£¬½«_____________L HClĘųĢåČÜÓŚ1000 mLĖ®ÖŠ(Ė®µÄĆܶČĪŖ1 g/cm3)£¬ĖłµĆČÜÖŹÖŹĮæ·ÖŹżĪŖ36.5%µÄŃĪĖį”£(±£ĮōŠ”ŹżµćŗóŅ»Ī»)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äźø£½ØŹ”øßČżÉĻѧʌµŚŅ»“ĪŌĀæ¼»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
£Ø7·Ö£©ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅŃÖŖ24æĖAŗĶ40æĖBĒ”ŗĆĶźČ«·“Ӧɜ³É0.4molCŗĶ32æĖD£¬ŌņCµÄĦ¶ūÖŹĮæĪŖ ”£
£Ø2£©°Ń1 molNaŗĶ1mol
Mg·Ö±šĶ¶Čėµ½¹żĮæµÄŃĪĖįÖŠ,·Ö±šµĆµ½ČÜŅŗaŗĶb,ŌņČÜŅŗaŗĶbµÄÖŹĮæ¹ŲĻµĪŖ

£Ø3£©ÓŅĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæµÄ±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż£¬ŹŌøł¾Ż±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż»Ų“š
ĻĀĮŠĪŹĢā£ŗ

¢ŁøĆÅØŃĪĖįÖŠHClµÄĪļÖŹµÄĮæÅضČĪŖ mol/L”£
¢Ś±ź×¼×“æöĻĀ£¬1.00LĖ®£ØĆÜ¶Č£ŗ1.00g”¤cm£3£©ĪüŹÕ LµÄHClæÉÖʵĆÉĻŹöÅØŃĪĖį”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com