
��ҵ����������ʱ�����ô�������Ӧ��SO2 ת��ΪSO3��һ���ؼ����衣ѹǿ���¶ȶ�SO2ת���ʵ�Ӱ�����±���ԭ�������ɷֵ��������Ϊ��SO2 7% ��O2 11%��N2 82%����
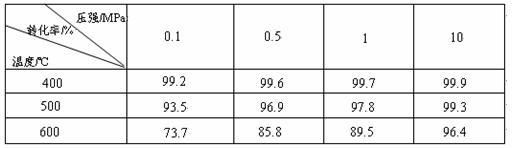
��1����֪SO2�������Ƿ��ȷ�Ӧ��������ñ��������ƶϴ˽��ۣ�
��
��2���ڴ�400��500��ʱ��SO2�Ĵ��������ó�ѹ�����Ǹ�ѹ����Ҫԭ���ǣ�
��
��3��ѡ�����˵Ĵ������Ƿ�������SO2��ת���ʣ� ����ǡ������Ƿ��������÷�Ӧ���ų��������� ����ǡ�����
��4��Ϊ���SO3�����ʣ�ʵ���������� ����SO3��
��5����֪��2SO2(g)+O2(g)��2SO3(g)����H����196.6kJ��mol��1������ÿ����1���98%��������Ҫ��SO3��������SO2������ЩSO3���ų���������
��1��ѹǿһ��ʱ,�¶�����ʱ,SO2ת�����½�,˵�������������淴Ӧ����,��������ӦΪ���ȷ�Ӧ��
��2������ѹǿ�����SO2ת����������Ӱ�죬���������ӳɱ���
��3���� ��
��4��Ũ����
��5���⣺1���98%�����ẬH2SO4��������9.8��109g
����ҪSO3������Ϊx���÷�Ӧ����������Ϊy��
H2SO4����������SO3������������H
98g 80g -196.6��0.5KJ
9.8��109g x y
x=![]() ��8.0��10��9g��8.0��103t
��8.0��10��9g��8.0��103t
y=![]() =9.83��109kJ
=9.83��109kJ
�������ɱ��е����ݣ���ѹǿ��ͬ�����������¶ȣ�SO2��ת���ʽ��ͣ�ƽ�������ƶ�������ӦΪ���ȷ�Ӧ��������ѹǿʱSO2��ת���ʱ仯�����ҳ�ѹ��ת�����ѽϴ�ʹ�ô����ӿ췴Ӧ���ʣ�ƽ�ⲻ�ƶ���ת���ʲ��䣬Ҳ��������Ӧ�ų���������Ϊ�μӷ�Ӧ����û�仯��Ϊ�˷�ֹ�γ�����������SO3���գ�ʵ������ŨH2SO4������SO3����1���98%������H2SO4����������SO3�������ų�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com