
£Ø8·Ö£©Li-SOC12µē³ŲæÉÓĆÓŚŠÄŌąĘš²«Ę÷£¬øƵē³ŲµÄµē¼«²ÄĮĻ·Ö±šĪŖļ®ŗĶĢ¼£¬µē½āŅŗŹĒLiAlCl4”ŖSOCl2.µē³ŲµÄ×Ü·“Ó¦æɱķŹ¾ĪŖ£ŗ4Li+2SOC12  4LiCl +S+SO2”£
4LiCl +S+SO2ӣ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)µē³ŲµÄøŗ¼«²ÄĮĻĪŖ_________£¬·¢ÉśµÄµē¼«·“Ó¦ĪŖ____________________£»
(2) SOCl2Ņ×»Ó·¢£¬ŹµŃéŹŅÖŠ³£ÓĆNaOHČÜŅŗĪüŹÕSOC12£¬Čē¹ū°ŃÉŁĮæĖ®µĪµ½SOCl2ÖŠ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________________________________£»
(3)ÓĆ“ĖŠīµē³Ųµē½āŗ¬ÓŠ0.1 mol/LCuSO4ŗĶ0.1 mol/LNaClµÄ»ģŗĻČÜŅŗ100 mL£¬¼ŁČēµēĀ·ÖŠ×ŖŅĘĮĖ0.02 mole-£¬ĒŅµē½ā³ŲµÄµē¼«¾łĪŖ¶čŠŌµē¼«£¬Ńō¼«²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żŹĒ______L£¬½«µē½āŗóµÄČÜŅŗ¼ÓĖ®Ļ”ŹĶÖĮ1L£¬“ĖŹ±ČÜŅŗµÄpH=______________”£
£Ø1£©Li Li-e-=Li+£ØĆææÕ1·Ö£©
£Ø2£©SOCl2+H2O=2HCl”ü+SO2”ü(2·Ö)
£Ø3£©0.168 2£ØĆææÕ2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©·ÖĪöµē³ŲµÄ×Ü·“Ó¦ÖŖ£¬øĆ·“Ó¦ÖŠLiµÄ»ÆŗĻ¼ŪÓÉ0¼ŪÉżøßµ½+1¼Ū£¬·¢ÉśŃõ»Æ·“Ó¦£¬×÷µē³ŲµÄøŗ¼«£¬µē¼«·“Ó¦Ź½ĪŖLi-e-=Li+£»£Ø2£©SOCl2ÓėĖ®·“Ӧɜ³ÉSO2ŗĶHCl£¬·“Ó¦µÄ·½³ĢŹ½ĪŖSOCl2+H2O=2HCl”ü+SO2”ü£»£Ø3£©0.1 mol/LCuSO4ŗĶ0.1 mol/LNaClµÄ»ģŗĻČÜŅŗ100 mLŗ¬ÓŠ0.01molCuSO4ŗĶ0.01molNaCl£¬µ±µēĀ·ÖŠ×ŖŅĘĮĖ0.02 mole-Ź±£¬½įŗĻĄė×ӵķŵēĖ³ŠņÖŖ£¬Ńō¼«µē¼«·“Ó¦ŅĄ“ĪĪŖ£ŗ2Cl--2e-=Cl2”ü£¬4OH--4e-=2H2O+O2”ü£¬ĄūÓƵē×ÓŹŲŗć½įŗĻĢāøųŹż¾Ż¼ĘĖćµĆÉś³ÉĀČĘų0.005mol£¬Éś³ÉŃõĘų0.0025mol£¬ĖłŅŌŃō¼«ÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż=£Ø0.005mol+0.0025mol£©”Į22.4L/mol=0.168L£»Ńō¼«ĒāŃõøłĄė×Ó¼õÉŁ0.01mol£¬ŌņČÜŅŗÖŠĒāĄė×ÓŌö¼Ó0.01mol£¬½«µē½āŗóµÄČÜŅŗ¼ÓĖ®Ļ”ŹĶÖĮ1L£¬ČÜŅŗÖŠĒāĄė×ÓÅضČĪŖ0.01mol/L£¬ČÜŅŗPH=2”£
æ¼µć£ŗæ¼²éŌµē³ŲŌĄķŗĶµē½āŌĄķµÄÓ¦ÓĆ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŌŚÓĆZnʬ”¢CuʬŗĶĻ”ĮņĖį×é³ÉµÄŌµē³Ų×°ÖĆÖŠ£¬¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
| A£®ŠæʬŹĒÕż¼«£¬ĶʬÉĻÓŠĘųÅŻ²śÉś | B£®µēĮ÷·½ĻņŹĒ“ÓŠæʬĮ÷ĻņĶʬ |
| C£®ČÜŅŗÖŠĮņĖįµÄĪļÖŹµÄĮæÅØ¶Č¼õŠ” | D£®µē½āÖŹČÜŅŗµÄpH±£³Ö²»±ä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø¹²10·Ö£©ĻÖÓŠŃōĄė×Ó½»»»Ä¤”¢ŅõĄė×Ó½»»»Ä¤”¢ŹÆÄ«µē¼«£¬ĒėÓĆĀČ¼ī¹¤ŅµÖŠµÄĤ¼¼ŹõŌĄķ£¬»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©Š“³öĀČ¼ī¹¤ŅµÖŠµē½ā±„ŗĶŹ³ŃĪĖ®µÄĄė×Ó·½³ĢŹ½ £»
£Ø2£©ĒėĄūÓĆ½»»»Ä¤¼¼Źõ£¬øł¾ŻÉĻĶ¼æņ¼Ü£¬Éč¼ĘŅ»øöµē½āĮņĖįÄĘČÜŅŗÖĘĒāŃõ»ÆÄĘČÜŅŗŗĶĮņĖįČÜŅŗµÄ×°ÖĆ£¬±ź³ö½ų³öĪļÖŹµÄ»ÆѧŹ½£ŗ
A_________£»C_________£» E_________£»Ä¤bĪŖ Ąė×Ó½»»»Ä¤£ØĢī”°Ńō”±»ņ”°Ņõ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼¼×”¢ŅŅŹĒµē»ÆѧŹµŃé×°ÖĆ”£
£Ø1£©Čō¼×”¢ŅŅĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠNaClČÜŅŗ£¬Ōņ£ŗ
¢Ł¼×ÖŠŹÆÄ«°ōÉĻµÄµē¼«·“Ó¦Ź½ĪŖ £»
¢ŚŅŅÖŠ×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»
¢Ū½«ŹŖČóµÄµķ·ŪKIŹŌÖ½·ÅŌŚŅŅÉÕ±ÉĻ·½£¬·¢ĻÖŹŌÖ½Ļȱ䥶ŗóĶŹÉ«£¬ÕāŹĒŅņĪŖ¹żĮæµÄCl2Ńõ»ÆĮĖÉś³ÉµÄI2 ”£Čō·“Ó¦ÖŠCl2ŗĶI2µÄĪļÖŹµÄĮæÖ®±ČĪŖ5”Ć1£¬ĒŅ Éś³ÉĮ½ÖÖĖį£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©Čō¼×ŅŅĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠ×ćĮæCuSO4ČÜŅŗ£¬Ōņ£ŗ
¢Ł¼×ÖŠĢś°ōÉĻµÄµē¼«·“Ó¦Ź½ĪŖ £»
¢ŚČē¹ūĘšŹ¼Ź±ŅŅÖŠŹ¢ÓŠ200mLpH=5µÄCuSO4ČÜŅŗ(25”ę)£¬Ņ»¶ĪŹ±¼äŗóČÜŅŗµÄpH±äĪŖ1£¬ČōŅŖŹ¹ČÜŅŗ»Öø“µ½µē½āĒ°µÄדĢ¬£¬æÉĻņČÜŅŗÖŠ¼ÓČėµÄĪļÖŹŹĒ (Š“»ÆѧŹ½)£¬ĘäÖŹĮæĪŖ g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)”¾»Æѧ”Ŗ”Ŗ»ÆѧÓė¼¼Źõ”æ
ŗ£Ė®Õ¼µŲĒņ×Ü“¢Ė®ĮæµÄ97£®2£„”£Čō°Ńŗ£Ė®µ»ÆŗĶ»Æ¹¤Éś²ś½įŗĻĘšĄ“£¬¼ČæÉŅŌ½ā¾öµĖ®×ŹŌ“ȱ·¦µÄĪŹĢā£¬ÓÖæÉŅŌ³ä·ÖĄūÓĆŗ£Ńó׏Ō“”£
(1)¶ą¼¶ÉĮÕō·ØŹĒÄæĒ°”°ŗ£Ė®µ»Æ”±µÄÖ÷ŅŖ¼¼Źõ”£øĆ·ØŹĒŌŚŅ»¶ØĢõ¼žĻĀ½«ŗ£Ė®±ä³ÉÕōĘū£¬ÕōĘū¾¹żĄäČ“¶ųµĆøß“æ¶ČµĖ®”£ÓÉ“ĖæÉÅŠ¶Ļ¶ą¼¶ÉĮÕō·ØŹĒ (Ģī”°ĪļĄķ±ä»Æ”± »ņ”°»Æѧ±ä»Æ”±)”£
(2)ĄūÓĆŗ£Ė®É¹ŃĪµÄŌĄķŹĒ £»·ÖĄėŹ³ŃĪ¾§ĢåŗóµÄÄøŅŗÖŠŗ¬ÓŠKCl”¢MgCl2£¬¾¹ż·ÖĄė”¢Ģį“æŗó£¬æÉÓĆÓŚ ”£
(3)”°ĀČ¼ī¹¤Ņµ”±ĄūÓƵē½ā±„ŗĶŹ³ŃĪĖ®ÖʵĆÖŲŅŖ»Æ¹¤²śĘ·”£ŌŚĀČ¼ī¹¤ŅµÖŠ£¬øōĤ·Øµē½ā(ČēĶ¼¼×ĖłŹ¾)¹¤ŅÕÖš½„±»Ąė×Ó½»»»Ä¤µē½ā(ČēĶ¼ŅŅĖłŹ¾)¼¼ŹõČ”“ś”£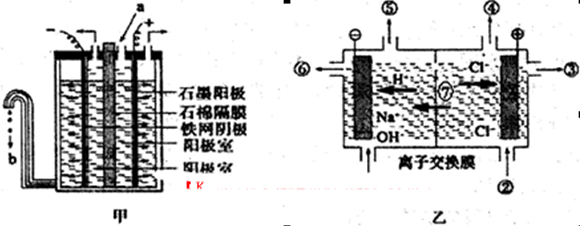
¢ŁŠ“³öĮ½µē¼«µÄ·“Ó¦Ź½£ŗŃō¼« £¬Ņõ¼« ”£
¢ŚŹÆĆŽøōĤµÄ×÷ÓĆŹĒ ”£Ąė×Ó½»»»Ä¤µē½ā²ŪÖŠ¢Ž”¢¢ß·Ö±šŹĒ ”¢ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĪŖ»ŲŹÕĄūÓĆ·Ļ·°“߻ƼĮ£Øŗ¬ÓŠV2O5”¢VOSO4¼°²»ČÜŠŌ²ŠŌü£©£¬æĘŃŠČĖŌ±×īŠĀŃŠÖĘĮĖŅ»ÖÖĄė×Ó½»»»·Ø»ŲŹÕ·°µÄŠĀ¹¤ŅÕ£¬Ö÷ŅŖĮ÷³ĢČēĻĀ£ŗ
²æ·Öŗ¬·°ĪļÖŹŌŚĖ®ÖŠµÄČܽāŠŌČēĻĀ£ŗ
| ĪļÖŹ | VOSO4 | V2O5 | NH4VO3 | £ØVO2£©2SO4 |
| ČܽāŠŌ | æÉČÜ | ÄŃČÜ | ÄŃČÜ | Ņ×ČÜ |

 VO2+£«H2O£«V3+£¬µē³Ų³äµēŹ±Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
VO2+£«H2O£«V3+£¬µē³Ų³äµēŹ±Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĒāŃõČ¼ĮĻµē³ŲµÄŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£¬»Ų“šĻĀĮŠÓŠ¹ŲøĆŹµŃéµÄĪŹĢā”£
£Ø1£©ŅŖŹ¹·¢¹ā¶ž¼«¹Ü·¢ĮĮ£¬ŹµŃ鏱µÄ²Ł×÷Ė³ŠņŹĒ£ŗĻČ_______£¬µē½āŅ»¶ĪŹ±¼äŗó£¬__________£»
£Ø2£©ŌŚŹµŃéµÄČ«¹ż³ĢÖŠÄÜĮæµÄÖ÷ŅŖ×Ŗ»ÆŠĪŹ½ŹĒ £»
£Ø3£©Š“³öĻĀĮŠĮ½ÖÖ²Ł×÷Ź±µÄµē¼«·“Ó¦Ź½£¬²¢×¢Ć÷µē¼«Ćū³Ę£ŗ
¢Ł°“ĻĀæŖ¹ŲS1£¬¶ĻæŖæŖ¹ŲS2£¬Ņ»¶ĪŹ±¼äŗó£ŗ C1ĪŖ ¼«£¬µē¼«·“Ó¦£ŗ £»
¢Ś¶ĻæŖæŖ¹ŲS1£¬ŃøĖŁ°“ĻĀæŖ¹ŲS2£ŗ£ØĢįŹ¾£ŗ“ĖŹ±C1ø½½üČÜŅŗĻŌ¼īŠŌ£¬C2ø½½üČÜŅŗĻŌĖįŠŌ£©C2ĪŖ______¼«£¬ µē¼«·“Ó¦£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©£Ø¢ń£©ĻĀĮŠ×°ÖĆÄÜ×é³ÉŌµē³ŲµÄŹĒ_____________ £ØĢīŠņŗÅ£©
£Ø¢ņ£©ČēÉĻĶ¼¢ÜĖłŹ¾£¬ČōŹĒŌµē³Ų£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ØČō²»ŹĒ£¬Ōņ²»ÓĆ»Ų“š£©”£
£Ø1£©Õż¼«²ÄĮĻŹĒ_______________________
£Ø2£©øŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ________________________
£Ø3£©µē³Ų¹¤×÷Ź±×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ____________________________
£Ø4£©ŅõĄė×ÓµÄŅĘ¶Æ·½Ļņ___________________________________
£Ø5£©µ¼ĻßÖŠµē×ÓµÄĮ÷Ļņ________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øÖĢśŗÜČŻŅ×ÉśŠā¶ų±»øÆŹ“£¬ĆæÄźŅņøÆŹ“¶ųĖšŹ§µÄøÖĢśÕ¼ŹĄ½ēøÖĢśÄź²śĮæµÄĖÄ·ÖÖ®Ņ»”£Ēė»Ų“šøÖĢśŌŚøÆŹ“”¢·Ą»¤¹ż³ĢÖŠµÄÓŠ¹ŲĪŹĢā”£
(1)ĻĀĮŠÄÄøö×°ÖĆæÉ·ĄÖ¹Ģś°ō±»øÆŹ“________________”£

(2)ŌŚŹµ¼ŹÉś²śÖŠ£¬æÉŌŚĢś¼žµÄ±ķĆę¶ĘĶ·ĄÖ¹Ģś±»øÆŹ“”£×°ÖĆŹ¾ŅāĶ¼ČēĶ¼”£Ēė»Ų“š£ŗ
¢ŁAµē¼«¶ŌÓ¦µÄ½šŹōŹĒ________(Š“ŌŖĖŲĆū³Ę)£¬Bµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ________________________”£
¢Ś¶Ę²ćĘĘĖšŗ󣬶ĘĶĢś±Č¶ĘŠæĢśøüČŻŅ×±»øÆŹ“£¬Ēė¼ņŅŖĖµĆ÷ŌŅņ
____________________________________________________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com