
 ��I�����в����ᵼ��ʵ����ƫ�ߵ���
��I�����в����ᵼ��ʵ����ƫ�ߵ���


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �ӵ�ʳ������ʳ�������ӵ���أ���ʳ�κ���������I���㣩����20��50mg/kg�������������Σ�������Լ��ʳ�κ������DZ��ϼӵ�ʳ��������һ����Ҫ������ijѧ����ʵ���Ҳⶨʳ�þ������Ƿ⼰�ⶨ����������Ҫ��ӳΪ��
�ӵ�ʳ������ʳ�������ӵ���أ���ʳ�κ���������I���㣩����20��50mg/kg�������������Σ�������Լ��ʳ�κ������DZ��ϼӵ�ʳ��������һ����Ҫ������ijѧ����ʵ���Ҳⶨʳ�þ������Ƿ⼰�ⶨ����������Ҫ��ӳΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�����в����ᵼ��ʵ����ƫ�ߵ���
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߡ�
?B��������һ�����ʵ���Ũ����Һʱ���� 10 mL����Ͳ��ȡ5.0 mLҺ������ʱ���Ӷ���
?C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
?D��10%�������90%�����������������50����������Һ
?E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
?��������������������Ʒ��a������̨������Ȧ�����У� b����ƿc.�ζ��� d���ձ������ɣ� e�������� f.��ͷ�ι� g��������ƽ�������룩 h����ֽ i����Ͳ j��©�� k���¶ȼơ�
? ���������Լ���A��NaOH���� B��̼������Һ C���Ȼ�þ��Һ D������ˮ������գ�
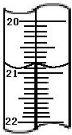
?��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ����������߿̶ȣ�����ȷ����
��
 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL
?B��������Ͳ������Ϊ2.5mL
?C�����ǵζ��ܣ�����Ϊ2.50mL
?D�������¶ȼƣ�������2.5��
?��2������һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������
?��3����ȥ Mg��OH��2�л��е����� Ca��OH��2�������õ��Լ��ǣ� ��ѡ����ţ������������� �� ϴ�ӡ��������������������������Ʒ�õ����� ����������������Ʒ��ѡ��������Ӧ��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ������ѧ�ڵ�һ������Բ��Ի�ѧ�� ���ͣ�ʵ����
��1�����в����ᵼ��ʵ����ƫ�ߵ���
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߡ�
?B��������һ�����ʵ���Ũ����Һʱ���� 10 mL����Ͳ��ȡ5.0 mLҺ������ʱ���Ӷ���
?C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
?D��10%�������90%�����������������50����������Һ
?E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
?��������������������Ʒ��a������̨������Ȧ�����У� b����ƿc.�ζ��� d���ձ������ɣ� e�������� f.��ͷ�ι� g��������ƽ�������룩 h����ֽ i����Ͳ j��©�� k���¶ȼơ�
? ���������Լ���A��NaOH���� B��̼������Һ C���Ȼ�þ��Һ D������ˮ������գ�
?��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ����������߿̶ȣ�����ȷ����
��
 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL
?B��������Ͳ������Ϊ2.5mL
?C�����ǵζ��ܣ�����Ϊ2.50mL
?D�������¶ȼƣ�������2.5��
?��2������һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������
?��3����ȥ Mg��OH��2�л��е����� Ca��OH��2�������õ��Լ��ǣ� ��ѡ����ţ������������� �� ϴ�ӡ��������������������������Ʒ�õ����� ����������������Ʒ��ѡ��������Ӧ��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ������ ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com