
������ԭ�ζ�ʵ��ͬ�к͵ζ�����(����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮)������0.001 mol��L��1KMnO4������Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ���ӷ���ʽ��2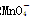 ��
��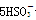 ��H��===2Mn2����5
��H��===2Mn2����5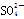 ��3H2O����ջش����⣺
��3H2O����ջش����⣺
(1)�õζ�ʵ�����������������е�________(�����)��
| A����ʽ�ζ���(50 mL) | B����ʽ�ζ���(50 mL) |
| C����Ͳ(10 mL) | D����ƿ |
(1)ABDEFH
(2)����������ǿ�������ܸ�ʴ��
(3)����ָʾ������Ϊ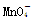 Mn2��ʱ��ɫ��ȥ
Mn2��ʱ��ɫ��ȥ
(4)�١�С
�������������(1)��Ϊ������ԭ�ζ�ʵ���������к͵ζ������к͵ζ�ʵ������������ѡ�ý���Ǩ�ƿɵó���ȷ�𰸡�
(2)����KMnO4����ǿ�����ԣ��ܸ�ʴ�ܣ��ʲ����ü�ʽ�ζ���ʢ��KMnO4��Һ��
(3)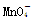 Ϊ��ɫ��Mn2��Ϊ��ɫ��������һ���Ե���ɫ�仯���жϵζ��յ㡣
Ϊ��ɫ��Mn2��Ϊ��ɫ��������һ���Ե���ɫ�仯���жϵζ��յ㡣
(4)�ζ�����Һ�棬�������ƫС������Ũ�ȱ�ʵ��Ũ��ƫС��
���㣺����ζ�ʵ����жϡ������Լ��йص�������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�������������


 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I.�й��к͵ζ��IJ����У� ���ñ���Һ��ϴ�ζ��� �����ζ�����ע�����Һ �ۼ��ζ����Ƿ�©ˮ �ܵζ� ��ϴ��
��ȷ�IJ���˳���� ������ĸ��
A. �ݢ٢ڢۢ� B. �ۢݢ٢ڢ� C. �ݢڢۢ٢� D. �ڢ٢ۢݢ�
��.������ԭ�ζ�ʵ��ͬ�к͵ζ����ƣ�����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮��������0.001mol��L��1KMnO4������Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ�����ӷ���ʽ�ǣ�2MnO4����5HSO3����H�� = 2Mn2����5SO42����3H2O
��ջش��������⣺
��1������ �����ʽ����ʽ�����ζ���ʢ�Ÿ��������Һ��
��2��ѡ����ָʾ�� ��������ţ�
�ټ��� �ڷ�̪ �� ʯ�� �ܲ���ָʾ��
��3���ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL����
��b��a��mL��ʵ������KMnO4��Һ��� ����ࡱ���١��������ݣ�b��a��mL����õ��Ĵ���ҺŨ�ȣ���ʵ��Ũ�� �����С������
��4��������KMnO4��Һ��NaHSO3��Һ�еΣ�����NaHSO3��Һ��KMnO4��Һ�еΣ�������������ȷ�淶��ǰ���£����в���һ����ʹ�ⶨ���ƫ�ߵ��� ��������ţ�
��ȡ��NaHSO3��Һ�ĵζ���δ�ô���NaHSO3��Һ��ϴ
��ȡ��KMnO4������Һ�ĵζ���δ�ñ�KMnO4������Һ��ϴ
�۵ζ������в�����Һ�彦��
�ܵζ�ǰ���Ӷ������ζ���ƽ�Ӷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ�����а��ظ߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ����
��1���ռ��ڱ�����̻Ჿ�ֱ��ʣ�������ҪΪNa2CO3����
ȷ��ȡ5.0g��Ʒ���Ƴ�250mL��Һ����ȡ���ƺõ��ռ���Һ10.00mL����ƿ�У��ֱ�����ƿ�и�����1��2�η�ָ̪ʾ��������֪����̪��ɫʱ����ʱֻ��NaOH��HCl��Ӧ��Na2CO3��û����HCl��Ӧ����Ũ��Ϊ0.20mol��L-1�������Һ���еζ���������ݼ�¼���£�
|
ʵ���� |
V���ռ���Һ��/mL |
V�����ᣩ/mL |
|
|
��ʼ���� |
ĩβ���� |
||
|
1 |
10.00 |
0.50 |
21.52 |
|
2 |
10.00 |
1.00 |
21.98 |
|
3 |
10.00 |
0.20 |
24.80 |
�Իش�
�ٵζ�ʱ���ֵ���ȷ������______________________________________��
���жϵ���ζ��յ��ʵ��������__________________________________________�����ݱ������ݣ�������ռ���Ʒ�к�NaOH����������Ϊ______ _����
�����в����ᵼ���ռ���Ʒ��NaOH�����ⶨֵƫ�ߵ���__________��
A����ƿ������ˮϴ��δ�ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
C���ζ�����������ƿʱ��������������Һ����
D���ζ�ǰƽ�Ӷ������ζ��������Ӷ���
��2��������ԭ�ζ�ʵ��ͬ�к͵ζ����ơ�������������Ҫ��������������ˮ��Һ�ֳ�Ϊ˫��ˮ��������������ɱ����Ư�ȡ�ij��ѧ��ȤС��ȡһ�����Ĺ���������Һ��ȷ�ⶨ�˹��������Ũ�ȡ���֪��2KMnO4��5H2O2��6H2SO4===2MnSO4��8H2O��5O2��������д���пհף�
���� (���ʽ����ʽ��)�ζ�����ȡ����������Һ25.00 mL����ƿ�У�������������
�ڵζ�ʱ����������ر���Һע����ʽ�ζ����У�������ر���Һ����ʽ�ζ���ԭ��Ϊ �����ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й�����������ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��³�ư���л�ѧѡ��6 3.2 ������ij��ֺ����IJⶨ��ϰ���������棩 ���ͣ������
������ԭ�ζ�ʵ��ͬ�к͵ζ�����(����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮)������0.001 mol��L��1����KMnO4��Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ���ӷ���ʽ��2MnO4-��5HSO3-��H��===2Mn2����5SO42-��3H2O����ջش����⣺
(1)�õζ�ʵ�����������������е�________��
A����ʽ�ζ���(50 mL) B����ʽ�ζ���(50 mL)
C����Ͳ(10 mL) D����ƿ
E������̨ F���ζ��ܼ�
G���ձ� H����ֽ
I����ͷ�ι� J��©��
(2)������________(��ᡱ�)ʽ�ζ���ʢ�Ÿ��������Һ���Է���ԭ��________________________________________________________________________
__________________________��
(3)ѡ����ָʾ����˵������
________________________________________________________________________
__________________________��
(4)�ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL����(b��a) mL��ʵ������KMnO4��Һ���________(��ࡱ���١�)������(b��a) mL����õ��Ĵ���Ũ�ȣ���ʵ��Ũ��________(���С��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��6 3.2 ���ʺ����IJⶨ��ϰ���������棩 ���ͣ������
������ԭ�ζ�ʵ��ͬ�к͵ζ�����(����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮)������0.001 mol��L��1KMnO4������Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ���ӷ���ʽ��2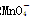 ��
�� ��H��===2Mn2����5
��H��===2Mn2����5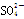 ��3H2O����ջش����⣺
��3H2O����ջش����⣺
(1)�õζ�ʵ�����������������е�________(�����)��
A����ʽ�ζ���(50 mL) B����ʽ�ζ���(50 mL)
C����Ͳ(10 mL) D����ƿ
E������̨ F���ζ��ܼ�
G���ձ� H����ֽ
I����ͷ�ι� J��©��
(2)����________(��ᡱ�)ʽ�ζ���ʢ�Ÿ��������Һ���Է���ԭ��
________________________________________________________________________��
(3)ѡ����ָʾ����˵������
________________________________________________________________________��
(4)�ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL����(b��a)mL��ʵ������KMnO4��Һ���ƫ________(��ࡱ���١�)������(b��a) mL����õ��Ĵ���Ũ�ȣ���ʵ��Ũ��ƫ________(���С��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������и߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
(����)������ԭ�ζ�ʵ��ͬ�к͵ζ����ƣ�����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮��������0.001mol/L���ԣ�MnO4��δ֪Ũ�ȵ���ɫ��aHSO3��Һ����Ӧ���ӷ���ʽ�ǣ�MnO4- + 5HSO3- + H+ ==2Mn2+ + 5SO42- + 3H2O ��ջش����⣺
�������õζ�ʵ�����������������е�______________________________(�����)
������ʽ�ζ��� �£���ʽ�ζ��� �ã���Ͳ �ģ���ƿ �ţ�����̨ �ƣ��ζ��ܼ� �ǣ��ձ� �ȣ���ֽ �ɣ���ͷ�ι� �ʣ�©��
��2���ζ�ǰƽ��KMnO4��Һ���̶�Ϊa mL�����ζ����յ㣩����Һ��̶�Ϊb mL����b-a��mL��ʵ������KMnO4��Һ���_________( �ࡢ��)������(b-a)mL����õ��Ĵ���ҺŨ�ȱ�ʵ�ʵ�Ũ��_________����С����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com