
�Ʊ�����þ��װ��ʾ��ͼ���£�

�ش��������⣺
(1)���װ�������Եķ�����____________��a��������________��b��������________��
(2)д��NaNO2��(NH4)2SO4��Ӧ�Ʊ������Ļ�ѧ����ʽ_____________________________��
(3)C��������________��D��������________���Ƿ����C��D��λ�öԵ���˵������________________________________________________________________________��
(4)д��E�з�����Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
(5)���û�ѧ����ȷ���Ƿ��е���þ���ɣ��������Ƿ���δ��Ӧ��þ��д��ʵ�����������________________________________________________________________________
________________________________________________________________________��
�𰸡�(1)��b����ʱG��������ð����ֹͣ������ȴ��G�в�����Һ��IJ������γ�һ���ȶ���ˮ���������������á���Һ©����Բ����ƿ
(2)(NH4)2SO4��2NaNO2��,2N2����Na2SO4��4H2O
(3)��ȥ����(����������)����ȥˮ���������ܣ��Ե�������ȥˮ����
(4)N2��3Mg��,Mg3N2
(5)ȡ�����������Թ��У�������������ˮ���Թܵײ��г������ɣ����ŵ��̼���ζ(���ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ���ֽ����)��֤�������к��е���þ����ȥ�ϲ���Һ���������ᣬ���۲쵽�����ݲ�������֤�������к���δ��Ӧ��þ
������(1)���װ��������ʱҪ��װ�����ó��ܱ���ϵ(�رշ�Һ©����������G�м���ˮ)��Ȼ������������ԭ�������顣
(2)NaNO2��NԪ���ԣ�3�ۣ��ڷ�Ӧ�н�(NH4)2SO4�У�3�۵����������߷������з�Ӧ����N2�����ݵ����غ�������غ�����ƽ��ѧ����ʽ��
(3)���ڿ�ʼװ���к��п���������������������ȥ�����е�O2����Ӧ�����ɵĵ������Ũ���������������ˮ���������ɵ�N2���
(4)þ�ǻ��ý������ڼ������������뵪����Ӧ���ɵ���þ��
(5)Mg3N2����ˮ��Ӧ����NH3����ֻҪ�������ˮ���ܷ����ɰ�������֪�Ƿ���Mg3N2���ɣ�����þ�ļ������ͨ�������������顣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
п��100 mL 18.5 mol·L��1��Ũ�����ַ�Ӧ��п��ȫ�ܽ⣬ͬʱ���������33.6 L(��״��)������Ӧ�����Һϡ����1 L�������Һ��pH��1��������������ȷ����(����)
A����Ӧ�й�����1.8 mol H2SO4
B���������SO2��H2�������Ϊ4��1
C����Ӧ�й�����97.5 g Zn
D����Ӧ�й�ת��1.5 mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A������������Ⱦ����Էֽ⣬�������NH3
B������������ֽⱬը��ʵ���ҳ��ü����Ȼ�����������ƵĹ��������Ʊ�����
C����ʢ�����������[(NH4)2Fe(SO4)2]��Һ���Թ��У��μ�����NaOH��Һ�����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬��ֽ����
D����ζ�������ˮ����ˮ��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����ģ��ϳɰ��Ͱ����������������£�


��֪ʵ���ҿ��ñ�����������(NaNO2)��Һ�뱥���Ȼ����Һ�����Ⱥ�Ӧ��ȡ������
(1)��ͼ��ѡ����ȡ����ĺ���װ�ã�����______________������______________��
(2)����������ͨ����װ�ã���װ�õ����ó��˽��������⣬����________��_________��
(3)���ϳ�����������ȴ����������ͨ����װ�õ�ˮ�����հ���________(���ᡱ���ᡱ)����������ԭ����______________________________________________________��
(4)����װ������һ��ʱ�䰱����ͨ�������ͬʱ�������ȵIJ�˿������װ�õ���ƿ�ڣ���ʹ��˿���ֺ��ȵ�ԭ����____________________________________________��
��ƿ�л��ɹ۲쵽��������________________________________________________��
(5)д����װ���а������Ļ�ѧ����ʽ��____________________________________
________________________________________________________________________��
(6)��Ӧ��������ƿ�ڵ���Һ�к��е�����ΪH����OH����________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ����һ�����������м���һ�����12 mol·L��1�����ᣬ���ȳ�ַ�Ӧ������������ϵ��һ���������ڵ���(����)
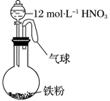
��NO ����Fe3������H������NO����NO2
����Fe3������H������NO����NO2
A���� B���٢�
C���ڢܢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D�Ƕ�����Ԫ���γɵ��������嵥�ʡ�E��F��Ϊ���壬��FΪ����ɫ���йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ)��
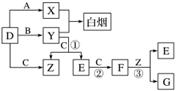
��ش��������⣺
(1)D�Ļ�ѧʽΪ________��
(2)��Ӧ�۵����ӷ���ʽΪ________________________________________��
(3)Y��E��һ�������¿ɷ�Ӧ����B��Z�����ø÷�Ӧ������E�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________________________________��
(4)0.1 mol·L��1��X��Һ��0.1 mol·L��1��Y��Һ�������ϣ���Һ��________��(��ᡱ��������С�)��ԭ����________________(�����ӷ���ʽ˵��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���У�����ȡ�ķ��뷽�����Ӧԭ������ȷ����(����)
| ѡ�� | Ŀ�� | ���뷽�� | ԭ�� |
| A | ��������ˮ�ĵ� | �Ҵ���ȡ | �����Ҵ��е��ܽ�Ƚϴ� |
| B | ���������������Ҵ� | ��Һ | �����������Ҵ����ܶȲ�ͬ |
| C | ��ȥKNO3�����л��ӵ�NaCl | �ؽᾧ | NaCl��ˮ�е��ܽ�Ⱥܴ� |
| D | ��ȥ�����е����� | ���� | ���������ѵķе����ϴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ϊ���ֵֽ����ࡢʪ����·���㳡����������ѧʽΪXY2����Һ�����¼���Xԭ�ӵĽṹʾ��ͼΪ ��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ��������������ǵ��Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӡ�
��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ��������������ǵ��Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӡ�
(1)m��________���ñ�ʪ���Ļ�ѧʽΪ________��
(2)Z��WԪ�ص�����Ϊ________��________��
(3)����˵����ȷ����________��
A��XY2��WZ2��Ϊ���ӻ�����
B��XY2�н������Ӽ���WZ2�н������ۼ�
C��H2Z��HY���ȶ���ǿ
D��X�������ӱ�Y�������Ӱ뾶��
(4)���л�ѧ���������ȷ����________��
A��XY2�ĵ���ʽ��X2��[


 ]2��
]2��
B��WZ2�Ľṹʽ��Z===W===Z
C��YԪ�صĵ�����H2Zˮ��Һ��Ӧ�����ӷ���ʽΪY2��Z2��===2Y����Z��
D���õ���ʽ��ʾXY2���γɹ���Ϊ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com