
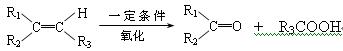
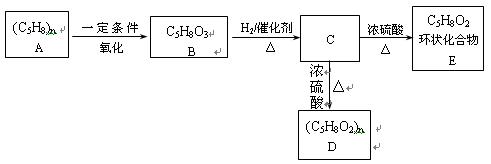
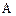 ��������__________��
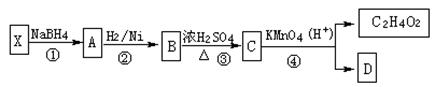
������Ϊ��__________�� �ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________��
�ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________�� ת��Ϊ
ת��Ϊ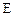 �Ļ�ѧ����ʽ____________________��
�Ļ�ѧ����ʽ____________________��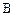 ��ͬ���칹��
��ͬ���칹��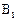 ������ֻ��һ���������ܷ���ˮ�ⷴӦ����
������ֻ��һ���������ܷ���ˮ�ⷴӦ����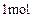
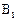 ������������Һ��Ӧ������ܵ�
������������Һ��Ӧ������ܵ�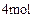
 ����
����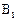 ���п��ܵĽṹ��ʽΪ��__________��
���п��ܵĽṹ��ʽΪ��__________��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ж���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��������������Ҫ�ɷֽṹ��ʽΪ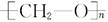 ����ͨ���Ӿ۷�Ӧ�Ƶõģ��䵥����HCHO ����ͨ���Ӿ۷�Ӧ�Ƶõģ��䵥����HCHO |
B���߷���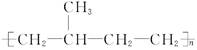 �ĵ����� �ĵ�����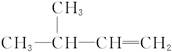 |
C��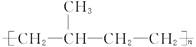 ����������ֱ�Ӵ����ͽṹ��Ϊ���ͽṹ ����������ֱ�Ӵ����ͽṹ��Ϊ���ͽṹ |
D�� ��������������ֱ�������ͽṹ������ͽṹ ��������������ֱ�������ͽṹ������ͽṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
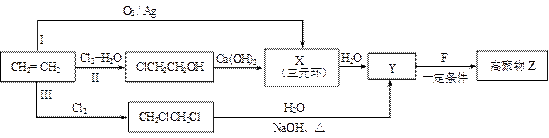
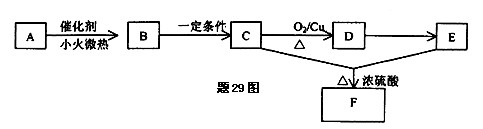
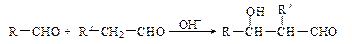 ��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��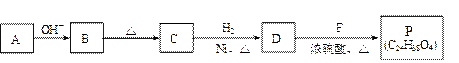
 ����ˮ��ΪM��N b��һ��������M����ת��ΪN
����ˮ��ΪM��N b��һ��������M����ת��ΪN�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
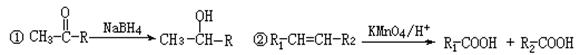
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
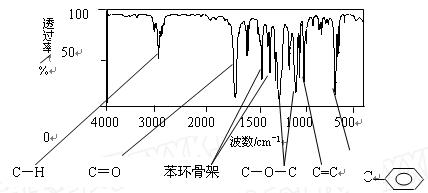
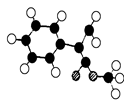
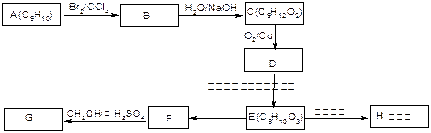
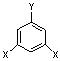 ��ʾ������X��Y����ΪH��
��ʾ������X��Y����ΪH��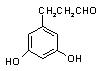 ��
��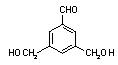 �� ��
�� �� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ���������
���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ��������� ���ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹΪ��
���ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹΪ��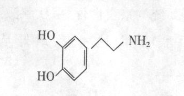
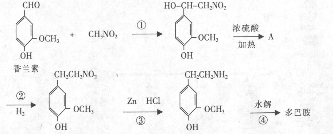
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com