
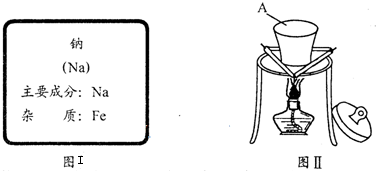
| ʵ����� | Ԥ�ڵ���������� | ��ػ�ѧ����ʽ |
| ȡ����ʵ��������Һ������ |
|
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?����ģ�⣩����ѧ�뼼����ģ��
��2011?����ģ�⣩����ѧ�뼼����ģ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ�� ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������С�⣬��16 �֡�
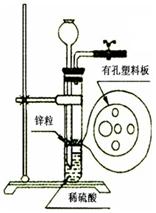
��ijͬѧ��ʵ����������һ������Zn����ͼ��ʾ��װ����ȡһ������H2����ʵ����������ϡ������Һ�����������������Һ�ɼ��룬����ϸ˼������ͨ������©������������ij�Լ������ʵ�顣�����Լ�����������ѡ�õ��ǣ���д���룩 ��
A��KCl��Һ B���ƾ� C���� D�����Ȼ�̼
���мס�����ѧ���ֱ�����IJ���̽�����⣺�����ʷ�����ѧ�仯��ǰ������
�����Ƿ������ӻ���С���߲���?
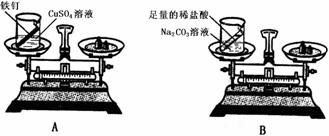
(1)������裺���ʷ�����ѧ�仯��ǰ�������������䡣
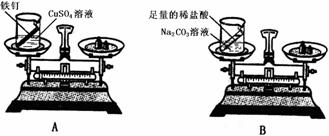
(2)��Ʒ���������ʵ�飺����Ƶ�ʵ��װ�ú�ѡ��ҩƷ����ͼA��ʾ������Ƶ�ʵ��װ�ú�
ѡ��ҩƷ����ͼB��ʾ�������ڷ�Ӧǰ�����˹淶�IJ�����ȷ�ij�����ϸ�µĹ۲졣
��ʵ����ۣ�
����Ϊ���ڻ�ѧ��Ӧ�У���������������뷴Ӧ�����������ȣ�
����Ϊ���ڻ�ѧ��Ӧ�У��������������뷴Ӧ������������ȡ�
������Ϊ �Ľ�����ȷ��
����Ӻ�۵ĽǶȷ�������Ϊ��ȷ�Ľ��۵�ԭ�� ��
������۵ĽǶȷ�������Ϊ��ȷ�Ľ��۵�ԭ�� ��
����д������ʵ�����йط�Ӧ�����ӷ���ʽ
A ��B ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������С�⣬��16 �֡�
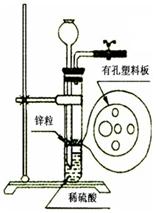
��ijͬѧ��ʵ����������һ������Zn����ͼ��ʾ��װ����ȡһ������H2����ʵ����������ϡ������Һ�����������������Һ�ɼ��룬����ϸ˼������ͨ������©������������ij�Լ������ʵ�顣�����Լ�����������ѡ�õ��ǣ���д���룩 ��
A��KCl��Һ B���ƾ� C���� D�����Ȼ�̼
���мס�����ѧ���ֱ�����IJ���̽�����⣺�����ʷ�����ѧ�仯��ǰ������
�����Ƿ������ӻ���С���߲���?
(1)������裺���ʷ�����ѧ�仯��ǰ�������������䡣
(2)��Ʒ���������ʵ�飺����Ƶ�ʵ��װ�ú�ѡ��ҩƷ����ͼA��ʾ������Ƶ�ʵ��װ�ú�
ѡ��ҩƷ����ͼB��ʾ�������ڷ�Ӧǰ�����˹淶�IJ�����ȷ�ij�����ϸ�µĹ۲졣
��ʵ����ۣ�
����Ϊ���ڻ�ѧ��Ӧ�У���������������뷴Ӧ�����������ȣ�
����Ϊ���ڻ�ѧ��Ӧ�У��������������뷴Ӧ������������ȡ�
������Ϊ �Ľ�����ȷ��
����Ӻ�۵ĽǶȷ�������Ϊ��ȷ�Ľ��۵�ԭ�� ��
������۵ĽǶȷ�������Ϊ��ȷ�Ľ��۵�ԭ�� ��
����д������ʵ�����йط�Ӧ�����ӷ���ʽ
A ��B ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com