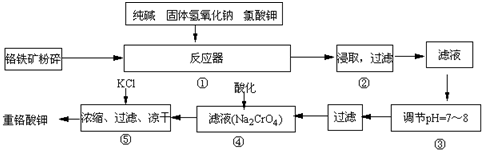
���ظ���أ�K
2 Cr
2O
7��ˮ��Һ�гȺ�ɫ��Cr
2O
72-���ɫ��CrO
42-������ƽ���ϵ��
Cr
2O
72-��aq��+H
2O��l��?2CrO
42-��aq��+2H
+��aq��
��1��д��������Ӧ��ƽ�ⳣ������ʽ��K=
| c2(CrO42-)?c2(H+) |
| c(Cr2O72-) |
| c2(CrO42-)?c2(H+) |
| c(Cr2O72-) |
��2�����ظ�����м�������NaOH���壬��Һ��
��
��
ɫ��
��3����2�����õ���Һ�м��������ϡ���ᣬ����Һ��
�Ⱥ�ɫ
�Ⱥ�ɫ
ɫ����Ϊ
�����ᣬH+Ũ������ƽ�����ƣ�Cr2O72-Ũ��������Һ�ʳȺ�ɫ
�����ᣬH+Ũ������ƽ�����ƣ�Cr2O72-Ũ��������Һ�ʳȺ�ɫ
��4����ԭ��Һ�м���Ba��NO
3��
2��Һ��Ba CrO
4Ϊ�����Ի�ɫ����������ƽ��
�����ƶ�
�����ƶ�
��������ƶ������������ƶ������ƶ���������Һ��ɫ��
��dz
��dz
��