
ijС�鰴ͼ1��ʾ��װ��̽������������ʴ�����������գ�


��1��ͼ2 ��ͼ1��ʾװ�õ�ʾ��ͼ����ͼ2��С��������д�������ϵĻ�ѧʽ���ڷ��������ü�ͷ��ʾ�����������ķ���
��2��д������������Ӧ�ķ���ʽ�������� �������� ��
��3����ͼ1װ��ʵ�飬Լ8���Ӳſ����ĵ�����Һ�����������д�ʩ���Ը���������ع۲쵽Һ���������� ��
a���ô����������֧�Թ��ڵĿ���
b����ʳ��ˮ���ݹ���������պȡ���ۺ�̿�۵Ļ����
c����ëϸ����ܴ��沣�����ܣ������Թܵ�ˮ�еμ�������īˮ
��4�������¶ȿ��Լӿ컯ѧ��Ӧ���ʣ������þƾ��Ƽ��Ⱦ�֧�Թܡ���һ��ʩ ������С����С�����
��5����ͬѧ�۲쵽ͼ1װ������װʱ�ͻ�ʹ������Һ������Թ���Һ�棬����ʵ��ʱ������Һ��������Ҫ�����ʱ�䡣ͼ1װ����װʱ��ʹ������Һ������Թ���Һ���ԭ���� ��������һ����ļ����� ��
��1�� ����1�֣���2�֣�
����1�֣���2�֣�
��2��2H2O+ O2+4e��4OH�� ��1�֣�Fe-2e��Fe2+��1�֣�
��3��a b c ��3�֣�
��4�����У�1�֣�
��5�������Ǻ������ӻ��Ǻ��ܲ���ˮ�У�����������������������С��ѹǿ����ʹ������Һ������Թ���Һ�棨2�֣�������Ͳ�����������������Ƥ�����ɴ���Һ©���ĵ�������2�֣����������֣�
��������
�����������1���Ȼ�����Һ�����ԣ�������ʳ��ˮ�У���������������ʴ������������̼�����������Ӵ����ص�����������̼����ͼ��Ϊ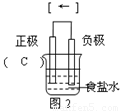
��2����װ���У���������ʧ���ӷ���������Ӧ���缫��ӦʽΪFe��2e����Fe2+�������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H2O+O2+4 e����4OH -��
��3��a���ô����������֧�Թ��ڵĿ�����������Ũ������Ӧ���ʼӿ죬��a��ȷ��b����ʳ��ˮ���ݹ���������պȡ���ۺ�̿�۵Ļ�������Ӧ��ĽӴ��������Ӧ���ʼӿ죬��b��ȷ��c����ëϸ����ܴ��沣�����ܣ������Թܵ�ˮ�еμ�������īˮ���ı���ͬ��ѹǿ���ı���ͬ�������ëϸ����������ĸ߶ȴ��ڲ������ܣ��Һ�īˮ��������ԣ���c��ȷ�����Դ�ѡa b c��
��4���¶�Խ�ߣ��������ܽ��ԽС����Ӧ����ԽС�������þƾ��Ƽ��Ⱦ�֧�Թܣ����ͷ�Ӧ���ʣ������һ��ʩ�Dz��еġ�
��5��һ���������壬ѹǿ����������ɷ��ȣ����ԽС��ѹǿԽ���µ�����Һ������Թ���Һ�棬���Կ��Բ�������Ͳ�����������������Ƥ�����ɴ���Һ©���ĵ������ķ���������һ�������ʹ������Һ������Թ���Һ���ԭ���Dz����Ǻ������ӻ��Ǻ��ܲ���ˮ�У�����������������������С��ѹǿ����ʹ������Һ������Թ���Һ�棻����������һ����ļ���������Ͳ�����������������Ƥ�����ɴ���Һ©���ĵ�������
���㣺����������������ʴԭ����ʵ��̽���Լ�ʵ�鷽����������۵�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
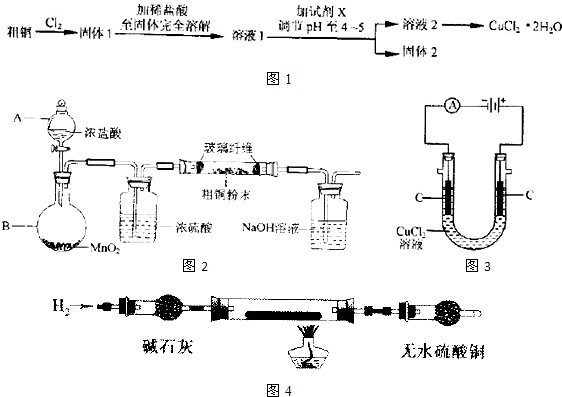
| ||
| ||
| c(CuCl 42- ) |
| c[Cu(H2O) 42- ]?c4(Cl-) |
| c(CuCl 42- ) |
| c[Cu(H2O) 42- ]?c4(Cl-) |
| ||
| ||
�鿴�𰸺ͽ���>>
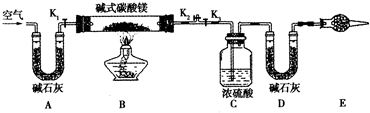
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
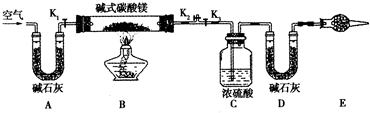
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ��ʦ��һ���и߿���ѧģ���Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com