
ЗжЮі ЃЈ1ЃЉОпгаЯрЭЌКЫЕчКЩЪ§МДжЪзгЪ§ЕФЭЌвЛРрдзгЕФзмГЦНадЊЫиЃЛОпгавЛЖЈЪ§ФПжЪзгКЭвЛЖЈЪ§ФПжазгЕФвЛжждзгНазіКЫЫиЃЌОпгаЯрЭЌжЪзгЪ§ЃЌВЛЭЌЕФжазгЪ§ЕФКЫЫиЛЅГЦЭЌЮЛЫиЃЌЖдгкдзгAZXРДЫЕЃЌжазгЪ§ЃЈNЃЉ=жЪСПЪ§ЃЈZЃЉ-жЪзгЪ§ЃЈAЃЉЃЛ
ЃЈ2ЃЉдкбєРызгжаЃЌКЫЕчКЩЪ§=жЪзгЪ§=КЫЭтЕчзгЪ§+ЫљДјЕчКЩЪ§ЃЛдквѕРызгжаЃЌКЫЕчКЩЪ§=жЪзгЪ§=КЫЭтЕчзгЪ§-ЫљДјЕчКЩЪ§ЃЌОнДЫЗжЮіЃЛ
ЃЈ3ЃЉвЛАуРДЫЕЃЌЛюЦУН№ЪєКЭЛюЦУЗЧН№ЪєдЊЫижЎМфвзаЮГЩРызгМќЃЌВЛЭЌЗЧН№ЪєдЊЫижЎМфвзаЮГЩМЋадМќЃЌЭЌжжЗЧН№ЪєдЊЫижЎМфвзаЮГЩЗЧМЋадМќЃЌКЌгаРызгМќЕФЛЏКЯЮяЪЧРызгЛЏКЯЮяЃЌжЛКЌЙВМлМќЕФЛЏКЯЮяЪЧЙВМлЛЏКЯЮяЃЌОнДЫЗжЮіНтД№ЃЛ
ЃЈ4ЃЉЂйИљОнЭЌвЛжїзхдЊЫиЕФзюЭтВуЕчзгЪ§ЯрЕШЃЛ
ЂкЕк3жмЦкЕФзюИпЛЏКЯМлДгзѓЁњгввРДЮЩ§ИпЃЛ
ЂлМюН№ЪєЪєгкН№ЪєОЇЬхЃЌАыОЖдНДѓЃЌН№ЪєМќдНШѕЃЎ
НтД№ НтЃКЃЈ1ЃЉдзгЃК${\;}_{6}^{12}$CЁЂ${\;}_{7}^{14}$NЁЂ${\;}_{11}^{23}$NaЁЂ${\;}_{1}^{3}$HЁЂ${\;}_{92}^{235}$UЁЂ${\;}_{19}^{40}$KЁЂ${\;}_{92}^{238}$UжаЃЌ${\;}_{92}^{235}$UЁЂ${\;}_{92}^{238}$UжЪзгЪ§ЖМ92ЃЌЪєгкЭЌвЛдЊЫиЃЌЫљвдЙВга6жждЊЫиЃЌОпгавЛЖЈЪ§ФПжЪзгКЭвЛЖЈЪ§ФПжазгЕФвЛжждзгНазіКЫЫиЃЌЫљвдЙВга7жжКЫЫиЃЌдзгAZXЃЌжазгЪ§ЃЈNЃЉ=жЪСПЪ§ЃЈZЃЉ-жЪзгЪ§ЃЈAЃЉЃЌ${\;}_{92}^{235}$UЁЂ${\;}_{92}^{238}$UжЪзгЪ§ЖМ92ЃЌжазгЪ§ВЛЭЌЃЌЪєгкЭЌЮЛЫиЃЌ
ЙЪД№АИЮЊЃК6ЁЂ7ЁЂ${\;}_{92}^{235}$UЁЂ${\;}_{92}^{238}$UЃЛ
ЃЈ2ЃЉИљОндквѕРызгжаЃККЫЕчКЩЪ§=жЪзгЪ§=КЫЭтЕчзгЪ§-ЫљДјЕчКЩЪ§ЃЌМДAXn-КЫЕчКЩЪ§=жЪзгЪ§=x-nЃЌгжИљОнжЪСПЪ§=жЪзгЪ§+жазгЪ§ЃЌМДAXn-жазгЪ§=жЪСПЪ§-жЪзгЪ§=A-ЃЈx-nЃЉЃЌ
ЙЪД№АИЮЊЃКA-x+nЃЛ
ЃЈ3ЃЉNH3жЛКЌМЋадМќЃЌЪєгкЙВМлЛЏКЯЮяЃЛNa2OжЛКЌРызгМќЃЌЪєгкРызгЛЏКЯЮяЃЛCO2жЛКЌМЋадМќЃЌЪєгкЙВМлЛЏКЯЮяЃЛ
CaCl2жЛКЌРызгМќЃЌЪєгкРызгЛЏКЯЮяЃЛCCl4жЛКЌМЋадМќЃЌЪєгкЙВМлЛЏКЯЮяЃЛH2O2КЌМЋадМќЁЂЗЧМЋадМќЃЌЪєгкЙВМлЛЏКЯЮяЃЛN2жЛКЌЗЧМЋадМќЃЌЪєгкЙВМлЛЏКЯЮяЃЛ
NaHCO3жаКЌгаРызгМќКЭМЋадМќЃЌЪєгкРызгМќЛЏКЯЮяЃЛNaOHжаКЌгаРызгМќКЭМЋадМќЃЌЪєгкРызгМќЛЏКЯЮяЃЛNH4ClжаКЌгаРызгМќКЭМЋадМќЃЌЪєгкРызгМќЛЏКЯЮяЃЛ
Na2O2жаКЌгаРызгМќКЭЗЧМЋадМќЃЌЪєгкРызгМќЛЏКЯЮяЃЌ
ЙЪД№АИЮЊЃКNa2O2ЃЛ
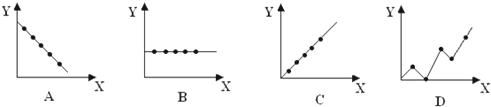
ЃЈ4ЃЉЂйЂђAзхдЊЫиЕФзюЭтВуЕчзгЪ§ЯрЕШЃЌЭМЯѓBЗћКЯЃЌ
ЙЪД№АИЮЊЃКBЃЛ
ЂкЕк3жмЦкЕФзюИпЛЏКЯМлДгзѓЁњгввРДЮЩ§ИпЃЌЭМЯѓCЗћКЯЃЌ
ЙЪД№АИЮЊЃКCЃЛ
ЂлвђМюН№ЪєЪєгкН№ЪєОЇЬхЃЌАыОЖдНДѓЃЌН№ЪєМќдНШѕЃЌШлЗаЕудНЕЭЃЌдђМюН№ЪєЕЅжЪЕФШлЕуЫцдзгађЪ§ЕФдіДѓЖјНЕЕЭЃЌЭМЯѓAЗћКЯЃЌ
ЙЪД№АИЮЊЃКAЃЎ
ЕуЦР БОЬтПМВщдзгЕФЙЙГЩЁЂЛЏбЇМќЁЂдЊЫижмЦкТЩЕШжЊЪЖЃЌБШНЯЛљДЁЃЌВржиЖдЛљДЁжЊЪЖЕФЙЎЙЬЃЎзЂвтЮяжЪжаДцдкЕФЛЏбЇМќЃЌзЂвтвЛжжВЛЭЌЕФдзгОЭЪЧвЛжжКЫЫиЃЌЬтФПФбЖШВЛДѓЃЎ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКбЁдёЬт
| AЃЎ | БљДзЫсЁЂДПМюЁЂУЂЯѕЁЂЩњЪЏЛвЗжБ№ЪєгкЫсЁЂМюЁЂбЮЁЂбѕЛЏЮя | |
| BЃЎ | HClOЁЂH2SO4ЃЈХЈЃЉЁЂHNO3ОљОпгаЧПбѕЛЏадЃЌЖМЪЧбѕЛЏадЫс | |
| CЃЎ | ЦЏАзЗлЁЂИЃЖћТэСжЁЂБљЫЎЁЂЭѕЫЎЁЂТШЫЎОљЮЊЛьКЯЮя | |
| DЃЎ | Na2OЃЌNaOHЃЌNaClЃЌNa2SO4ЃЌNa2O2ЖМЪєгкФЦЕФКЌбѕЛЏКЯЮя |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКбЁдёЬт
| AЃЎ | Ag+ЁЂNO3-ЁЂCl-ЁЂK+ | BЃЎ | Na+ЁЂFe2+ЁЂCl-ЁЂNO3- | ||
| CЃЎ | K+ЁЂBa2+ЁЂOH-ЁЂSO42- | DЃЎ | Cu2+ЁЂNH4+ЁЂBr-ЁЂCl- |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКНтД№Ьт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКбЁдёЬт
| AЃЎ | ШШЮШЖЈадЃКHFЃОHClЃОH2S | BЃЎ | дзгАыОЖЃКBrЃОSeЃОCl | ||
| CЃЎ | ЛЙдадЃКS2-ЃОSe2-ЃОCl- | DЃЎ | ЫсадЃКHBrO4ЃОHClO4ЃОH2SO4 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКбЁдёЬт
| AЃЎ | HCO3-ЁЂAg+ЁЂNO3-ЁЂNa+ | BЃЎ | Na+ЁЂCl-ЁЂCO32-ЁЂNO3- | ||
| CЃЎ | Fe3+ЁЂNa+ЁЂCl-ЁЂSO42- | DЃЎ | H+ЁЂCl-ЁЂCO32-ЁЂNH4+ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКНтД№Ьт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
 ЃЎ
ЃЎВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКбЁдёЬт
| AЃЎ | дкМзБНжаМгШыЩйСПЫсадИпУЬЫсМиШмвКЃЌеёЕДКѓЭЪЩЋЃЌе§ШЗЕФНтЪЭЪЧгЩгкВрСДгыБНЛЗЕФЯрЛЅгАЯьЃЌЪЙВрСДКЭБНЛЗОљвзБЛбѕЛЏ | |
| BЃЎ | гУКЫДХЙВеёЧтЦзМјБ№1-БћДМЁЂ2-БћДМ | |
| CЃЎ | МфЖўфхБННігавЛжжПеМфНсЙЙПЩжЄУїБНЗжзгжаВЛДцдкЕЅЫЋМќНЛЬцЕФНсЙЙ | |
| DЃЎ | УКЕФИЩСѓЪЧЯжНёЛёЕУЗМЯуЬўЕФжївЊЭООЖ |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com