
��ش��������⣺
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��______________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ______________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��

��Ӧ�ٵĻ�ѧ����ʽ��_____________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_____________________________��
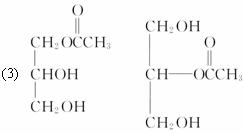
(3)C��B��������һ�������·�Ӧ���ɵĻ����������Ϊ134��д��C���п��ܵĽṹ��ʽ��________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������___________g�����ơ�
������(1)M������Cԭ�Ӹ���Ϊ��![]() ��3��Hԭ�Ӹ���Ϊ��
��3��Hԭ�Ӹ���Ϊ��![]() ��5;Nԭ�Ӹ���Ϊ
��5;Nԭ�Ӹ���Ϊ![]() ��3��Oԭ�Ӹ���Ϊ��
��3��Oԭ�Ӹ���Ϊ��![]() =9�����Է���ʽΪC3H5O9N3��DΪNO����Է�������Ϊ30��
=9�����Է���ʽΪC3H5O9N3��DΪNO����Է�������Ϊ30��
(2)��֬���Ǹ�֬���������������ˮ��õ���֬������ͣ����ͺ�HNO3���ո�������ʾ����������Ӧ���йط���ʽΪ��

(3)��1���Ӹ��ͺ�1����CH3COOH��Ӧ���������Է�������Ϊ134����1���Ӹ��ͺ�2����CH3COOH��Ӧ���������Է�������Ϊ176����1���Ӹ��ͺ�3����CH3COOH��Ӧ���������Է�������Ϊ218������C��1���Ӹ��ͺ�1����CH3COOH��Ӧ�IJ������ܵĽṹΪ��
(4)B�����Na��Ӧ��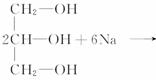
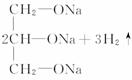
0.1molB����0.3molNa������Ϊ
�𰸣�(1)C3H5O9N3 NO
(2)��Ӧ�ٵĻ�ѧ����ʽ��
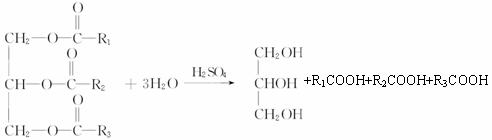
��Ӧ�ڵĻ�ѧ����ʽ��
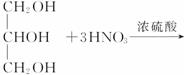
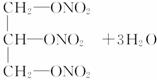
(4)6.9



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⣺
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��__________________________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ_______________________________��
(2)��֬A��ͼ2-3��ʾ;���ɵõ�M��

ͼ2-3
ͼ�Тڵ���ʾ��
C2H5OH+HO��NO2![]() C2H5O��NO2+H2O
C2H5O��NO2+H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��_____________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_____________________________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ��___________________________________________________________
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������___________________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⣺
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��__________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ__________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��
C2H5OH+HO��NO![]() C2H5O��NO2+H2O
C2H5O��NO2+H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��_________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ__________________________________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������___________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ________��
(2)��֬A������;���ɵõ�M��

ͼ8-6
ͼ�Тڵ���ʾ��
C2H5OH+HO��NO2![]() C2H5O��NO2+H2O
C2H5O��NO2+H2O
��Ӧ�ٵĻ�ѧ����ʽ��_______________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_______________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ_______________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�ҷ���ijҩ��M��������Ѫ�ܼ�������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�D����������D�������ڵ�����ԭ����Ϊ�������ٻ���1998��ŵ��������ѧ��ҽѧ����������������⡣
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��________________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ________________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��
C2H5OH��HO��NO2![]() C2H5O��NO2��H2O
C2H5O��NO2��H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��__________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��__________________________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ��________________________________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������_______________g�����ơ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com