
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
 Al��OH��3+3H+
Al��OH��3+3H+ Al��OH��3+3H+
Al��OH��3+3H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
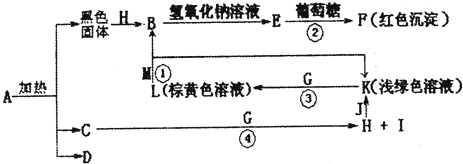
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������ʮ�������ص�ѧУ2012�������ҵ������(��)��ѧ���� ���ͣ�022
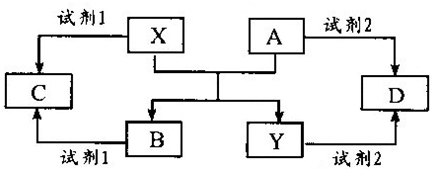
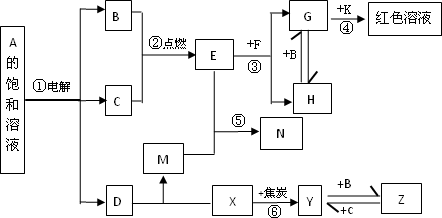
����A��Z����ͼ��ʾ��ת����ϵ(���ַ�Ӧ�����������ȥ)�����У�������B��CΪ���嵥�ʣ�YΪ���嵥�ʣ�Y�ǵ��ӹ�ҵ�г��õİ뵼����ϣ�F�Ǿ��д��Եĺ�ɫ���壮E��ˮ��Һ��M��Ϻ�õ�N��N����������ˮ�ɵõ�һ�ֳ�����ʵ���Һʹ�װʳƷ��ƿװʳƷ�ȵĸ������
��ش�
(1)K�Ļ�ѧʽ________��Z�ĵ���ʽ________��D�д��ڵĻ�ѧ������Ϊ________��
(2)��Ӧ�ٵ����ӷ���ʽ________����Ӧ�۵Ļ�ѧ����ʽ________��
(3)��H��ˮ��Һ�м���������ˮ������Һ¶���ڿ����У��۲쵽��������________��
(4)д����Ӧ�Ļ�ѧ����ʽ________���÷�Ӧ��ÿ����1 mol��X��ת�Ƶ���________mol��
�鿴�𰸺ͽ���>>
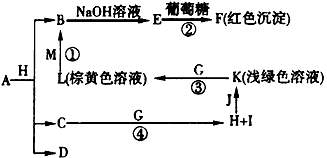
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�Իش���������:
(1)M�ĵ���ʽΪ:_______________��M��������������֮��Ϊ:_______________��
(2)G�ڷ�Ӧ�٣����зֱ���_______________����_______________����
(3)д�����з�Ӧ�Ļ�ѧ����ʽ:
A+B��C+D:______________________________��
D+M��L:________________________________��
F��G�������B��E:______________________________��
(4)д�����з�Ӧ�����ӷ���ʽ:
K+E+D��I+H:______________________________��
I+G��K+E+J:______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com