

 +CaCl2+H2O���÷�Ӧ��ԭ��������25.4%��
+CaCl2+H2O���÷�Ӧ��ԭ��������25.4%�� ��
�� �����Ҷ������ã�������һϵ�л����
�����Ҷ������ã�������һϵ�л����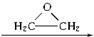 HO-CH2-CH2-O-CH2-CH2-OH
HO-CH2-CH2-O-CH2-CH2-OH
���� ��1��������ˮ��Ӧ�����Ȼ���ʹ����ᣬ�����ữΪ��ϩ�ʹ�����ļӳɷ�Ӧ������2-���Ҵ���
��2��2-���Ҵ����������Ʒ�Ӧ���ɻ������顢�Ȼ��ƺ�ˮ����������е�10.4�森��ˮ����������ܣ�ͨ������1����ķ�����û������飬�����������ѡ������������
��3���ȴ���Ϊ��ϩ�������ͼӦ�����ɻ���������Ȼ��ƺ�ˮ������ԭ��������=$\frac{Ԥ�ڲ����������}{ȫ����Ӧ���������}$��100%���㣻
��4����ϩ������������������һ�����ɻ��������ƣ���ɫ��ѧҪ��Ӧ��ԭ�Ӿ�����ת���������
��5�������л���Ľṹ��ʽ���ͨʽΪ��C2nH 4n+2On��Ȼ�����ü�������������������ֵ��
��� �⣺��1��������ˮ��Ӧ�����Ȼ���ʹ����ᣬ��Ӧ�����ӷ���ʽΪ��Cl2+H2O=H++Cl-+HClO���������ǹ��ۻ������ԭ������ԭ�ӡ���ԭ�ӷֱ�ͨ��1�Թ��õ��ӶԽ�ϣ��ṹʽΪH-O-Cl����ϩ�Ĵ����ữΪ��ϩ�ʹ�����ļӳɷ�Ӧ�������ܷ�Ӧ����ʽΪ��CH2=CH2+Cl2+H2O��ClCH2CH2OH+HCl��
�ʴ�Ϊ��CH2=CH2+Cl2+H2O��ClCH2CH2OH+HCl���ӳɷ�Ӧ��
��2����ϩ�Ĵ����ữ�������������ƣ�������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��2-���Ҵ����������Ʒ�Ӧ��ClCH2CH2OH+Ca��OH��2�� +CaCl2+H2O����������е�10.4�森��ˮ����������ܣ����ܵ�Һ������÷е�IJ�ͬ����������룬���Բ���1Ϊ�������������Ҫ�����������������飬����ƿ���ܻ������飬����ѡ��CE��
+CaCl2+H2O����������е�10.4�森��ˮ����������ܣ����ܵ�Һ������÷е�IJ�ͬ����������룬���Բ���1Ϊ�������������Ҫ�����������������飬����ƿ���ܻ������飬����ѡ��CE��
�ʴ�Ϊ������CE��
��3���ȴ���Ϊ��ϩ�������ͼӦ���������������Ʒ�Ӧ�����Ȼ��ơ�������ƺ�ˮ����ϩ�ʹ�����Ʒ�Ӧ���ɻ������飬�����ܷ�Ӧ����ʽΪ��CH2=CH2+Cl2+Ca��OH��2�� +CaCl2+H2O���÷�Ӧ��ԭ��������=$\frac{Ԥ�ڲ����������}{ȫ����Ӧ���������}$��100%=$\frac{44}{28+71+74}$��100%��25.4%��
+CaCl2+H2O���÷�Ӧ��ԭ��������=$\frac{Ԥ�ڲ����������}{ȫ����Ӧ���������}$��100%=$\frac{44}{28+71+74}$��100%��25.4%��
�ʴ�Ϊ��CH2=CH2+Cl2+Ca��OH��2�� +CaCl2+H2O��25.4%��
+CaCl2+H2O��25.4%��
��4����ϩ�����������������·�Ӧ��2CH2=CH2+O2$\stackrel{Ag}{��}$2 ���÷�Ӧͨ��һ���ӳɷ�Ӧ���ɣ�ԭ��������Ϊ100%������Ⱦ��
���÷�Ӧͨ��һ���ӳɷ�Ӧ���ɣ�ԭ��������Ϊ100%������Ⱦ��
�ʴ�Ϊ��2CH2=CH2+O2$\stackrel{Ag}{��}$2 ������Ⱦ��ԭ��������100%��
������Ⱦ��ԭ��������100%��
��5������ͬϵ��ͨʽ���Ƶ�����֪����һϵ�л������ͨʽΪC2nH 4n+2On����̼Ԫ�ص���������Ϊ$\frac{2n��12}{2n��12+4n+2+16n}$��100%��n������������ʱ��̼Ԫ������������������ֵ��$\frac{6}{11}$��54.54%��
�ʴ�Ϊ��B��
���� ���⿼���л��ﻷ������ļ����Ʊ�������ע����ݸ������Ϣ���л���ṹ�����ƶϣ����ؿ���ѧ���ķ���������������Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������ܽ�� | B�� | ��ֹ���������ֽ� | ||
| C�� | ������������ˮ�� | D�� | ϡ�����ֹ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
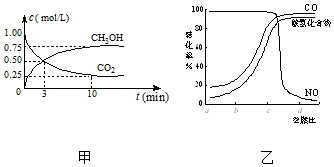 CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
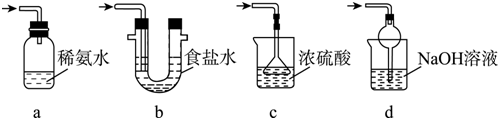
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
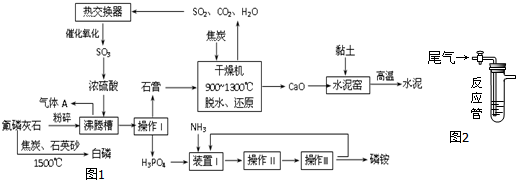
| ��� | �Լ��� | ���� |
| �� | NaOH��Һ����̪��Һ | |
| �� | Na2CO3��Һ����̪��Һ | |
| �� | ��ˮ��������Һ | |
| �� | KMnO4��Һ��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
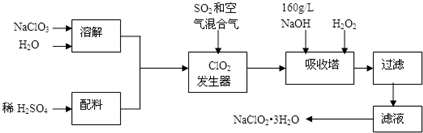
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
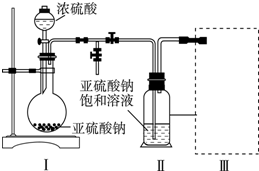
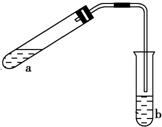 ������ͼ��ʾ��װ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ��������գ�
������ͼ��ʾ��װ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ��������գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2��SO2����Ʒ����Һ��ɫ��˵������Ư��ԭ����ͬ | |
| B�� | ŨNa2SO4��Һ�ͼ�ȩ����ʹ�����ʴ���Һ��������˵�����߾�ʹ�����ʷ������� | |
| C�� | CO2��SO2ʹ����ʯ��ˮ����ǣ�˵�����߾�Ϊ���������� | |
| D�� | ��Һ�еμ������ữ��Ba��NO3��2��Һ���ְ�ɫ������˵������Һ��һ����SO42- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com