
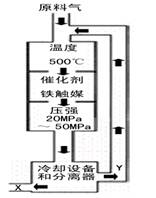
��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ���ԭ���ǣ�![]() ��H <0��������������ͼ��ʾ��
��H <0��������������ͼ��ʾ��
��X�Ļ�ѧʽΪ ��
����ͼ������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ� ��
A�������¶ȡ�����ѹǿ����������߰���ת����
B������ý�ڸ��¶�ʱ���Դ�
C����ҵ�����ܶ��������ϡ��豸������������
���������¶ȣ�����ƽ��ʱ��ƽ�ⳣ����__________(��������С��)��
�ܸı䷴Ӧ��������ʹƽ�ⷢ���ƶ�����ͼ��ʾ�������ı䣬�����İٷֺ����ı仯���ơ���������Ϊѹǿʱ���仯������ȷ���ǣ�ѡ����ĸ��ţ� ����������Ϊ�¶�ʱ���仯������ȷ���ǣ�ѡ����ĸ��ţ� ��

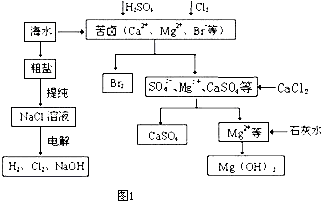
��2�������°�����������ˮ����ˮ��Һ���Ե��硣
���÷���ʽ��ʾ��������ˮ���������ԵĹ��̣�
��
�ް�ˮ��ˮ�������c(OH��) 10��7 mol��L-1����д����������������������
�߽���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ���Һ������Ũ���ɴ�С����Ϊ ��
��1��NH3 �� BC�ۼ�С��c����a��2����NH3 + H2O ![]() NH3��H2O
NH3��H2O ![]() NH4+ + OH- �� <���� c(Cl-) > c(NH4+) > c(H+) > c(OH-)
NH4+ + OH- �� <���� c(Cl-) > c(NH4+) > c(H+) > c(OH-)
���⿼�鹤ҵ�ϳɰ�����1���ںϳɰ���ҵ������ѡ������ѹǿ���ӿ췴Ӧ���ʣ���߰��IJ��ʣ������¶���Ҫ�ǿ��Ǵ����Ļ��ԣ�A����B��ȷ��ѹǿ��������Ҫ�����豸�ij���������C��ȷ���������¶ȣ�ƽ�������ƶ���ƽ�ⳣ����С��������ѹǿ��ƽ�������ƶ���������������c��ȷ�������¶ȣ�ƽ�������ƶ�������������С��a��ȷ����2���ް�ˮ���������OH������ˮ�ĵ��룻����ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ���ȫ��Ӧ�������Ȼ����Һ��NH4+ˮ��������ԣ�����Ũ���ɴ�СΪc(Cl-) > c(NH4+) > c(H+) > c(OH-)��


 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�Ϻ�һ��2010��2011ѧ��߶���ѧ�����п��Ի�ѧ���� ���ͣ�022
���ǵ����ϼ�Ϊ�ḻ��Ԫ�أ���д���пհף�
(1)NH![]() ��Nԭ�ӵ��ӻ��������Ϊ________��
��Nԭ�ӵ��ӻ��������Ϊ________��
NH![]() �Ŀռ乹��Ϊ________��
�Ŀռ乹��Ϊ________��
(2)�����£�﮿��뵪��ֱ�ӷ�Ӧ����Li3N��Li3N�����е���N3�����ڣ���̬N3���ĵ����Ų�ʽΪ��________��Li3N��������________����(�������)��
(3)NH3�ķе�Ϊ234K��NF3�ķе�Ϊ154K�����߽ṹ���ƣ�NH3�ķе����NF3��ԭ���ǣ�________��
(4)CO��N2���ƣ������ж�����һ������������CO��________���м����±�ΪCO��N2�������Ϣ�����ݱ������ݣ�˵��CO��N2���õ�ԭ��________��

(5)�����±����ݣ�д��������������Ӧ���ɰ������Ȼ�ѧ����ʽ________��
![]()
(6)CO��N2��Ϊ�ȵ����壬��̼ԭ������һ�Թ¶Ե��ӣ���˿������壬�磺Fe(CO)5��Ni(CO)4��Cr(CO)6�ȣ��ںϳɰ���ҵ������ͭϴҺ����CO����Ӧ���£�

�ٻ�̬Feԭ�ӵ�δ�ɶԵ�������________����
д��Cr��Cu+���۲������Ų�ʽ��________��________��
�ڴ��������ͭ(I)�ʹ����ʻ�������ͭ(I)��������[Cu(NH3)3CO]+���ṩ�¶Ե��ӵķ����ǣ�________�����ܹ¶Ե��ӵ������ǣ�________���ü��ű��[Cu(NH3)2]+�γɵ���λ����________��[Cu(NH3)2]+��������λ���ļ���Ϊ180�㣬��Cu+��ȡ________�ӻ���NH3�γ���λ��(���ӻ�����)��
��Ni(CO)4����ɫҺ�壬�е�42.1�棬�۵㣭19.3�棬������ˮ���������л��ܼ����Ʋ�Ni(CO)4��________����(����ԡ��Ǽ��ԡ�)��
(7)��������һ�ָ����մɲ��ϣ���Ӳ�ȴ��۵�ߡ���ѧ�����ȶ���
�ٵ����辧������________����(�������)��
����֪�����辧��ṹ�У�ԭ�Ӽ䶼�Թ��ۼ���������Nԭ����Nԭ�ӣ�Siԭ����Siԭ�Ӳ�ֱ��������ͬʱÿ��ԭ�Ӷ�����8���ӽṹ����д��������Ļ�ѧʽ��________��
(8)�����ĵ������ɵ�������(NaN3)���ȷֽ���õ���2NaN3(s)��2Na(l)��3N2(g)����Ӧ�����У����ѵĻ�ѧ�������Ӽ����ۼ����γɵĻ�ѧ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�γ��и�����ѧ�������Ի�ѧ���� ���ͣ������
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
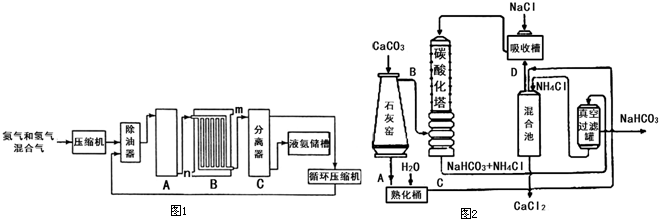
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5�� ����1��
����1�� ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS >CO2
>CO2
C����ͬѹǿ�·е㣺C2H 5SH>C2H5OH
5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4�� -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�
ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� �� ��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
| ��������/mol | 0.01 | 0.02 | 0.03 | 0.05 | 0.1 | 0.15 | 0.2 | 0.6 |
| ����% | 87.3 | 88.2 | 90.3 | 94.2 | 92.9 | 93.1 | 91.8 | 92.3 |
| ת����% | 89.7 | 92.1 | 93.9 | 98.9 | 94.9 | 95.7 | 93.9 | 94.3 |
| ��� | ȩ | �� | ת����% | ����% |
| 1 | ���ǻ�����ȩ | �״� | 94.3 | 89.6 |
| 2 | ���ǻ�����ȩ | �״� | 93.6 | 88.7 |
| 3 | ���ȱ���ȩ | �״� | 93.1 | 87.3 |
| 4 | ����������ȩ | �״� | 54.2 | 34.1 |
| 5 | ����������ȩ | �״� | 89.9 | 79.5 |
| 6 | ����������ȩ | �״� | 65.7 | 41.9 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣
��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣
��ҵ�ϳɰ�����ʾ��ͼ��ͼ1��ʾ��
��X�Ļ�ѧʽΪ ��
��ͼ������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ� ��
A���¶ȡ�ѹǿ�Ի�ѧƽ��Ӱ��
B������ý�ڸ��¶�ʱ���Դ�
C����ҵ�����ܶ��������ϡ��豸������������
�۸ı䷴Ӧ��������ʹƽ�ⷢ���ƶ���ͼ2��ʾ�������ı䣬
�����İٷֺ����ı仯���ơ���������Ϊѹǿʱ���仯������
ȷ���ǣ�ѡ����ĸ���ţ� ����������Ϊ�¶�ʱ���� ��������ȷ���ǣ�ѡ����ĸ��ţ� ��

��2�������°�����������ˮ����ˮ��Һ���Ե��硣
���÷���ʽ��ʾ��������ˮ�Ĺ����д��ڵĿ��淴Ӧ
��
��
�ڰ�ˮ��ˮ�������c(OH��) 10��7mol/L����д����������������������
�۽���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ���Һ������Ũ���ɴ���С����Ϊ ��
��3���������л�ԭ�ԣ���ͭ�Ĵ������£������ͷ�����Ӧ����A��B�������ʡ�AΪ��Σ�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75�����������ÿ��Ӧ8.96L��������״������ͬʱ����0.3molA��
��д�������ͷ�����Ӧ�Ļ�ѧ����ʽ ��
���ڱ�״���£�ÿ����1 mol B��ת�Ƶ��ӵ����ʵ���Ϊ mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com