
(14��)
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
��1���ڢٲ���Ӧ����ˮHF�������� �� ����Ӧ�豸�����ò������ʵ�ԭ���� (�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%�� ��Һ��ϴ��
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PCl5����ˮ�⣬�����Ϊ�����ᣬд��PCl5ˮ��Ļ�ѧ����ʽ�� ��
��3���ڢܲ�������õķ����� ���ڢݲ�����β����HF��HCl���õķ����� ��
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒwg�����Li�����ʵ���Ϊnmol�������Ʒ��LiPF6�����ʵ���Ϊ mol(�ú���w��n�Ĵ���ʽ��ʾ)��
��1����Ӧ�� �ܼ� SiO2+4HF=SiF4��+2H2O NaHCO3
��2��PF5+4H2O=H3PO4+5HF
��3������ ����
��4��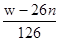
�����Ծ���������1������+Һ�������µ����ʼ�������Һ�����Ƿ�Ӧ����ܼ������ã���������Ҫ�ɷ�Ϊ�����κͶ������裬����HF��Ӧ��HF���������ԣ�������������к͡�
��2��PF5��PΪ+5�ۣ�FΪ-1�ۣ���ˮ��ΪH3PO4��HF��
��3����Һ�ķ�����ù��˵ķ�����HF��HCl�ķ��룬������е㲻һ����HF�д�������������з��룬�ʽ����¶ȣ�HF�ȱ�ΪҺ�壻
��4�������غ���Եõ�����LiPF6Ϊxmol��LiFΪymol������Li�غ㣬��x+y=n������������152x+26y=w�����Խ�á�
���㣺��ѧ���գ����ʵ����ʣ��������ᴿ����ѧ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ϳɰ���Ӧ��N2+3H2 2NH3+92.4KJ���ڻ�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ�����ʾ��ͼ��ͼ��ʾ��
2NH3+92.4KJ���ڻ�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ�����ʾ��ͼ��ͼ��ʾ��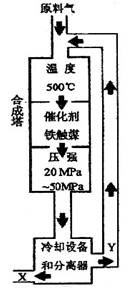
30. X�Ļ�ѧʽΪ ��X��״̬Ϊ_______̬��
31. ����ͼ����������˵����ȷ���� ����д��ţ���
a���ڴ��¶��£�������ƽ�������ƶ�������߰��IJ���
b������ý��ʹ��������ƽ�������ƶ�
c����ҵ�����ܶ��������ϡ��豸�����������ƣ�ѡ���ѹǿ
d��Ϊ���ԭ�ϵ�ת���ʣ�����ѭ������
32. �����°�����������ˮ����ˮ��Һ���Ե��硣
�� ��ˮ��ˮ�����ӻ���������ֵ��_______________________��
�� ����ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ�
��Һ������Ũ���ɴ�С����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ӻ�ˮ�Ʊ�����þ��������ͼ��ʾ��
��1��Ϊ�˽�Լ�ɱ���������ú�̲��Դ���ñ��Ǿ���һϵ�з�Ӧ�����Ƶ�ʯ���飬��д���йط�Ӧ�Ļ�ѧ����ʽ��__________��__________��
��2����ʯ�����м���MgCl2��Һ����ֽ��衢���ˡ�ϴ�ӡ�д���÷�Ӧ�Ļ�ѧ����ʽ��__________��
��3�����۵���Ӳ�ȷ���������þ�Ͻ���þ��Ƚϣ����ص���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������Ʒ��������Ӧ����
| A | B | C | D | ||
 ���� | 
|  ����� |  ������ | ||
| ���ǽ������� | �л��߷��Ӳ��� | �������� | ���ϲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ͼ�Ӳ�ʺϽ��к�̼���٣�WC���������ܣ�Co�������������������õ�ⷨ�ɻ���WC��Co���������̼�ͼ���£�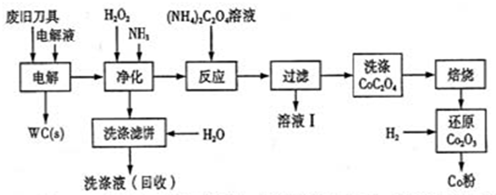
��1�����ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦΪ ��
��2���������������˱�����Ҫ�ɷ���Fe(OH)3�����յ�ϴ��Һ����ˮ���Ƶ��Һ��Ŀ���ǻ����������е� ��
��3����ҺI����Ҫ�ɷ���NH4Cl��ϴ��CoC2O4����ֶ����ղ�Ʒ���Ȳ�������Ӱ�죬������ʱ����ɻ�����Ⱦ��ԭ���� ��
��4����Co2O3��ԭ��Co�۵Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʯ�����ȼҵ�е�һ�ַ���������������±���ʾ��
�õ�ʯ����������ˮCaCl2��ij��������������¹������̣�
��֪�Ȼ��ƾ���Ļ�ѧʽ�ǣ�CaCl2��6H2O��H2S��һ���������壬�Ҿ��л�ԭ�ԡ�
��1����Ӧ���м������Ӧѡ��___________________��
��2����ɫ����Ӧ���������X��_______________���豸A��������______________���豸B������Ϊ________________���豸C��������____________________��
��3��Ϊ�����㻷��Ҫ���轫����H2Sͨ�����ճأ��������������ʺ���Ϊ���ռ�����_____________����Ӧ�Ļ�ѧ����ʽΪ_________________��
| A��ˮ | B��Ũ���� | C��ʯ���� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������أ�K2FeO4����һ�ּ�������������������һ������Ͷ��ˮ���������������������£�
��֪���� 2KOH + Cl2 =" KCl" + KClO + H2O���������¶Ƚϵͣ�
�� 6KOH + 3Cl2 =" 5KCl" + KClO3 + 3H2O���������¶Ƚϸߣ�
�� 2Fe(NO3)3 + 2KClO + 10KOH = 2K2FeO4 + 6KNO3 + 3KCl + 5H2O
�ش��������⣺
��1������������Ӧ�� ����¶Ƚϸߡ����¶Ƚϵ͡���������½��У�
��2��д����ҵ����ȡCl2�Ļ�ѧ����ʽ ��
��3��K2FeO4����Ϊ���Ͷ��ˮ��������ԭ�� ��
��4����MnO2 ��Zn������ƣ�K2FeO4 ��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ________���õ���ܷ�Ӧ�����ӷ���ʽΪ_____��
��5���ڡ���ӦҺI ���м�KOH�����Ŀ���Ǣ� ���� ��
��6���ӡ���ӦҺII ���з����K2FeO4����Ʒ�� ��д��ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ѣ�TiO2���㷺Ӧ���ڸ���ṹ����Ϳ�ϡ�ֽ��Ϳ��ȣ��������ѻ�����Ϊ�Ʊ��ѵ��ʵ�ԭ�ϡ�
�������ѿ����������ַ����Ʊ���
����1��TiCl4ˮ������TiO2��xH2O�����ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2���˷����Ʊ��õ��������������ѡ�
��1���� TiCl4ˮ������TiO2��x H2O�Ļ�ѧ����ʽΪ_______________________________ ��
�ڼ���TiO2��x H2O��Cl���Ƿ����ķ�����______________________________
����2�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�
��2���������ĩ�м����ᷴӦ��TiO2+�����ӷ���ʽΪ
��3����Һ���м���Fe��������
��4����Ҫ����FeSO4��7H2O�������ˮ�������������ƾ��ơ��������⣬��Ҫ�õ������ֹ�������������
��������ѿ�������ȡ�ѵ��ʣ��漰���IJ�������ͼ��
��Ӧ�ڵķ���ʽ�� ���÷�Ӧ��Ҫ��Ar�����н��У������ԭ�� _
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��SnSO4��һ����Ҫ�������Σ��㷺Ӧ���ڶ�����ҵ�����Ʊ��Ĺ����������£�
�ش��������⣺
��1�� SnCl2�����������ˮֱ���ܽ��ԭ���� ���������۵������� ��
��2�� ��ӦI���ɵij���ΪSnO��д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��3�� ��������Ѿ���ϴ�ӡ��ɾ��IJ����ǣ� ��
��4����ӦII���������֮һ�ǿ�����Һ��pH������Һ��c(Sn2+)=1.0mol��L��1����������Ӧ������ҺpH ������֪��Ksp[Sn(OH)2]=1.0��10��26��
��5�����������£�SnSO4��������˫��ˮ��ȥ��������д����������Ӧ�����ӷ���ʽ��
��
��6����ʪ�����У�����ͭ��ʹ��������Ҳ�ܷ�ֹ�γ�ͭ�̣������йص�ԭ��������ԭ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com