
 BaSO4”ż£«2H2O£»Ba2£«£«SO42£
BaSO4”ż£«2H2O£»Ba2£«£«SO42£ BaSO4”ż£»¢ĘH£«£«SO42££«Ba2£«£«OH£
BaSO4”ż£»¢ĘH£«£«SO42££«Ba2£«£«OH£ BaSO4”ż£«H2O£»H£«£«OH£
BaSO4”ż£«H2O£»H£«£«OH£ H2O£»¢Ē2Ba2£«£«4OH££«Al3+£«2 SO42£
H2O£»¢Ē2Ba2£«£«4OH££«Al3+£«2 SO42£ 2BaSO4”ż£«[Al£ØOH£©4]££¬3[Al£ØOH£©4]££«Al3+
2BaSO4”ż£«[Al£ØOH£©4]££¬3[Al£ØOH£©4]££«Al3+ 4 Al£ØOH£©3”ż”£
4 Al£ØOH£©3”ż”£ BaSO4”ż£«2H2O”£“ĖŹ±ÖŠŠŌČÜŅŗÖŠÖ»ÓŠNa2SO4ČÜŅŗ£¬¼ÓČėBa(OH)2µÄĄė×Ó·“Ó¦Ź½ĪŖBa2£«£«SO42£
BaSO4”ż£«2H2O”£“ĖŹ±ÖŠŠŌČÜŅŗÖŠÖ»ÓŠNa2SO4ČÜŅŗ£¬¼ÓČėBa(OH)2µÄĄė×Ó·“Ó¦Ź½ĪŖBa2£«£«SO42£ BaSO4”ż”£¢Ęµ±Ē”ŗĆ²»ŌŁÉś³É³ĮµķĪŖÖ¹Ź±µÄ·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖH£«£«SO42££«Ba2£«£«OH£
BaSO4”ż”£¢Ęµ±Ē”ŗĆ²»ŌŁÉś³É³ĮµķĪŖÖ¹Ź±µÄ·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖH£«£«SO42££«Ba2£«£«OH£ BaSO4”ż£«H2O£¬ŌČÜŅŗÉś³ÉNaOHČÜŅŗ£¬¼ĢŠųµĪ¼ÓNaHSO4ČÜŅŗ£¬Ö»·¢Éś£ŗH£«£«OH£
BaSO4”ż£«H2O£¬ŌČÜŅŗÉś³ÉNaOHČÜŅŗ£¬¼ĢŠųµĪ¼ÓNaHSO4ČÜŅŗ£¬Ö»·¢Éś£ŗH£«£«OH£ H2O£»¢Ēµ±ČÜŅŗÖŠBa2£«Ē”ŗĆĶźČ«³ĮµķŹ±µÄ·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ2Ba2£«£«4OH££«Al3+£«2 SO42£
H2O£»¢Ēµ±ČÜŅŗÖŠBa2£«Ē”ŗĆĶźČ«³ĮµķŹ±µÄ·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ2Ba2£«£«4OH££«Al3+£«2 SO42£ 2BaSO4”ż£«[Al£ØOH£©4]££¬ŌŁĻņČÜŅŗÖŠ¼ĢŠųµĪ¼ÓĆ÷·ÆČÜŅŗŹ±µÄ3[Al£ØOH£©4]££«Al3+
2BaSO4”ż£«[Al£ØOH£©4]££¬ŌŁĻņČÜŅŗÖŠ¼ĢŠųµĪ¼ÓĆ÷·ÆČÜŅŗŹ±µÄ3[Al£ØOH£©4]££«Al3+ 4 Al£ØOH£©3”ż”£
4 Al£ØOH£©3”ż”£

 ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®ĻņÉÕ¼īČÜŅŗÖŠĶØČė¹żĮæµÄ¶žŃõ»ÆĢ¼£ŗ2OH££«CO2====CO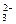 £«H2O £«H2O |
B£®Ļņ“æ¼īČÜŅŗÖŠĶØČė×ćĮæµÄ¶žŃõ»ÆĢ¼£ŗCO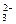 £«CO2£«H2O====2HCO £«CO2£«H2O====2HCO |
C£®ĻņĖ®²£Į§ÖŠĶØČė¶žŃõ»ÆĢ¼£ŗSiO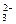 £«CO2£«2H2O====H4SiO4”ż£«CO £«CO2£«2H2O====H4SiO4”ż£«CO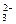 |
D£®Ļņ“ĪĀČĖįÄĘČÜŅŗÖŠĶØČė¶žŃõ»ÆĢ¼£ŗ2ClO££«CO2£«H2O====2HClO£«CO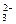 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®ÓĆŹÆÄ«µē¼«µē½āĻõĖįŅųČÜŅŗ£ŗ4Ag++4OH”Ŗ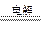 4Ag+ O2”ü+ 2H2O 4Ag+ O2”ü+ 2H2O |
| B£®Ģ¼ĖįĒāøĘČÜŅŗÖŠ¼ÓČė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£ŗHCO3”Ŗ+OH”Ŗ£½CO32-+ H2O |
| C£®“ĪĀČĖįøĘČÜŅŗÖŠĶØČĖÉŁĮæµÄ¶žŃõ»ÆĢ¼£ŗCa2++2C1O”Ŗ+H2O+CO2£½CaCO3”ż+ 2HClO |
| D£®ĮņĖįĆ¾ČÜŅŗÓėĒāŃõ»Æ±µČÜŅŗ»ģŗĻ£ŗBa2++ SO42££½BaSO4”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®“×ĖįÓėŠ”ĖÕ“ņČÜŅŗ·“Ó¦£ŗ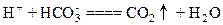 |
| B£®ĮņĒā»ÆÄʵÄĖ®½ā£ŗ HS-+H2O 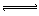 H3O++S2- H3O++S2- |
| C£®ĢśÓėČżĀČ»ÆĢśČÜŅŗ·“Ó¦£ŗ Fe+Fe3+====2Fe2+ |
| D£®ĀĮÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£ŗ |
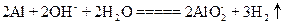
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÓĆ°±Ė®ĪüŹÕÉŁĮæSO2ĘųĢå£ŗ2NH3”¤H2O+SO2=2NH4++SO32-+H2O |
| B£®NaHSO4ČÜŅŗÖŠµĪČėBa(OH)2ČÜŅŗÖĮÖŠŠŌ£ŗH++SO42-+Ba2+Ź®OH-===BaSO4”ż+H2O |
| C£®ŌŚH2O2ÖŠ¼ÓČėĖįŠŌKMnO4ČÜŅŗ£ŗ2MnO4-+5H2O2+6H+====2Mn2+ +5O2”ü+8H2O |
D£®ÓƶčŠŌµē¼«µē½ā±„ŗĶĀČ»ÆÄĘČÜŅŗ£ŗ2Cl- + 2H+ H2”ü+ Cl2”ü H2”ü+ Cl2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 CO2”ü+H2OĄ“±ķŹ¾µÄŹĒ(””””)?
CO2”ü+H2OĄ“±ķŹ¾µÄŹĒ(””””)? | A£®Ģ¼Ėį±µÓėŃĪĖį·“Ó¦? |
| B£®Ģ¼Ėį¼ŲČÜŅŗÓėĻ”ĮņĖį·“Ó¦? |
| C£®ĖÕ“ņČÜŅŗÓėĻ”ĻõĖį·“Ó¦? |
| D£®Š”ĖÕ“ņČÜŅŗÓėĻ”ĮņĖį·“Ó¦? |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®°±ĘųĶØČė“×ĖįČÜŅŗCH3COOH+NH3=CH3COONH4 |
| B£®³ĪĒåµÄŹÆ»ŅĖ®øśŃĪĖį·“Ó¦H++OH-=H2O |
| C£®Ģ¼Ėį±µČÜÓŚ“×ĖįBaCO3+2H+=Ba2++H2O+CO2”ü |
| D£®½šŹōÄĘøśĖ®·“Ó¦2Na+2H2O=2Na++2OH-+H2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com