

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
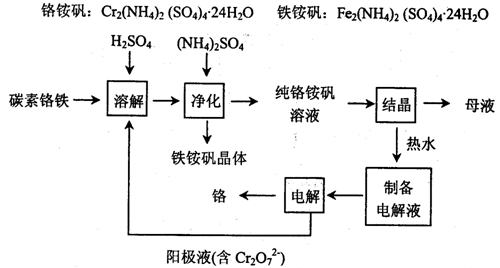
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢ� | B���٢� | C���٢ڢ� | D���ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
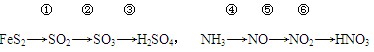
| A�����б仯��������-��ԭ��Ӧ | B���ڢۢݢ��ǻ��Ϸ�Ӧ |
| C�����������ж��нӴ���װ�� | D���ڢܷ�Ӧ��Ҫ�ô��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �� 400�桢30MPa��n(NH3)��n(H2)
�� 400�桢30MPa��n(NH3)��n(H2)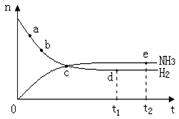
| A����a������Ӧ���ʱ�b��Ĵ� |
| B����c����Ӧ��δ�ﵽƽ�� |
| C����d��t1ʱ�̣��͵�e��t2ʱ�̣���n(N2)��һ�� |
| D�������������䣬500���·�Ӧ��t1ʱ�̣� n(H2)��ͼ��d���ֵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����ʱ������˫���꣨H2Dz����Ԫ���ᣩ�ѽ���������ϳɵ����Ե����ʣ�����CCl4��ȡ�����Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫���꣨H2Dz����CCl4������ˮ�е�Cu2��ʱ���ȷ�����Ϸ�Ӧ��
����ʱ������˫���꣨H2Dz����Ԫ���ᣩ�ѽ���������ϳɵ����Ե����ʣ�����CCl4��ȡ�����Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫���꣨H2Dz����CCl4������ˮ�е�Cu2��ʱ���ȷ�����Ϸ�Ӧ�� Cu(HDz)2+2H����
Cu(HDz)2+2H����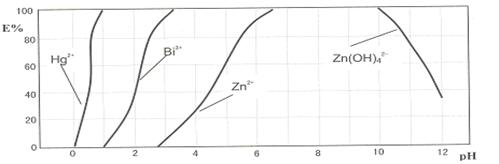
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
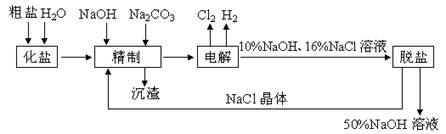
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
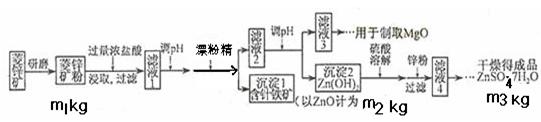
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com