
(9��)
2005��2��28��Ӣ��ʳƷ���������Ϲ����˺��ϡ�����������30����ҵ�����Ŀ��ܺ��С��յ���(һ��)��ʳƷ���嵥�Ժ�һ���Ѳ���˺����յ���(һ��)ʳƷ��ս����ȫ����Χ��չ�����յ���(һ��)��һ�ֺ�ɫɫ�أ������°����ã������͡����͡���������Ь�͵ȹ�ҵ����Ϊ���Ӽ�������������ʳƷ���յ���(һ��)�ģ�һ�ֺϳ�·�����£�

1��д���м���A��B��C�Ľṹʽ��(3��)
2��д�����Ӻ��յ���(һ��)�Ľṹʽ��(4��)
3���������ص�����£����ӷ���ż����ӦҲ����һ�ֺ�ɫȾ�ϨD�D��λ�죬�仯ѧ����Ϊ1��(4��������ż��)��2�����ӣ�д����λ��Ľṹʽ��(2��)

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����ϩ����ͨ������ϩ����ϩ���������ӳɷ�Ӧ�õ���

 ��BrHC=CHBr���������ӳɷ�Ӧ�õ�������A����֪
��BrHC=CHBr���������ӳɷ�Ӧ�õ�������A����֪ �Dz��ȶ��ġ�������ĿҪ����գ�
�Dz��ȶ��ġ�������ĿҪ����գ�

ͼ3-9
��1��ͨ������д��ç����ķ���ʽ______________________��
��2��д�������ӳ����ɻ�����A�ķ�Ӧ����ʽ______________________��
��3��D�����еĹ�����������______________________��
��4��д��D�ľ�����ͬ������ͬ���칹��Ľṹ��ʽ������д�������ȶ��ṹ��____________________________________________��
��5������ç�������������Ҵ���Ũ���������¼��ȣ��������ɷ���ʽΪC9H10O5���»�����÷�Ӧ�Ļ�ѧ����ʽΪ_____________________���䷴Ӧ����Ϊ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���������ʱ�������������ľ���Ħ�����������¡�Ϊ�˷�ֹ����¶ȹ��ߣ��ڻ������Ϳ��һ�������Ϳ�ϣ���Ϳ�ϵ���������ܵ���__________��?
A.�ڸ����²��ۻ�
B.�ڸ����¿ɷֽ�����?
C.�ڳ����¾ͷֽ�����
D.��Ϳ�ϲ����ܷ����ֽ�?
��2�����������Ҫ���ܵ�ȼ�ϣ���������N2O4��N2H4��Ϊȼ�ϣ��䷴Ӧ����Ϊ����Ⱦ���ʡ���д���÷�Ӧ�ķ���ʽ��______________���÷�Ӧ�б�������ԭ���뱻��ԭ��ԭ�����ʵ���֮����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�人����У����10��������ѧ�Ծ����������� ���ͣ������
(8��)�й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%~50%��
��1����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ������ܵ���
A�����ˮ���⣺2H2O 2H2����O2��
2H2����O2��
B������ʹˮ�ֽ����⣺2H2O 2H2����O2��
2H2����O2��
C��̫������ֽ�ˮ���⣺2H2O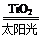 2H2����O2��
2H2����O2��
D����Ȼ�����⣺CH4��H2O CO��3H2
CO��3H2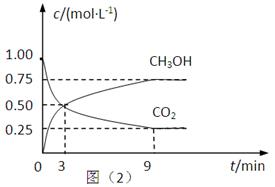
��2��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO2��3mol H2��һ�������·�Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
�ٴ�3 min��9 min��v(H2)=________mol��L��1��min��1��
����˵��������Ӧ�ﵽƽ��״̬����____________�����ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ��������3mol H2��ͬʱ����1mol H2O
D��CO2����������ڻ�������б��ֲ���
��3����ҵ�ϣ�CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO(g)+2H2(g) CH3OH(g)��ƽ�ⳣ����
CH3OH(g)��ƽ�ⳣ����
| �¶�/�� | 0 | 100 | 200 | 300 | 400 |
| ƽ�ⳣ�� | 667 | 13 | 1.9��10-2 | 2.4��10-4 | 1��10-5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�人����У����10��������ѧ�Ծ��������棩 ���ͣ������
(8��)�й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%~50%��
��1����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ������ܵ���
A�����ˮ���⣺2H2O 2H2����O2��
2H2����O2��
B������ʹˮ�ֽ����⣺2H2O 2H2����O2��
2H2����O2��
C��̫������ֽ�ˮ���⣺2H2O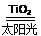 2H2����O2��
2H2����O2��
D����Ȼ�����⣺CH4��H2O CO��3H2
CO��3H2
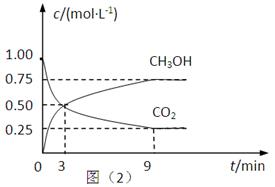
��2��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO2��3mol H2��һ�������·�Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
�ٴ�3 min��9 min��v(H2)=________mol��L��1��min��1��
����˵��������Ӧ�ﵽƽ��״̬����____________�����ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ��������3mol H2��ͬʱ����1mol H2O
D��CO2����������ڻ�������б��ֲ���
��3����ҵ�ϣ�CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO(g)+2H2(g) CH3OH(g)��ƽ�ⳣ����
CH3OH(g)��ƽ�ⳣ����
|
�¶�/�� |
0 |
100 |
200 |
300 |
400 |
|
ƽ�ⳣ�� |
667 |
13 |
1.9��10-2 |
2.4��10-4 |
1��10-5 |
����˵����ȷ����_____��
A���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ
B���÷�Ӧ�ڵ����²����Է����У������¿��Է����У�˵���÷�Ӧ��S��0
C����T��ʱ��1L�ܱ������У�Ͷ��0.1mol CO��0.2 mol H2���ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D����ҵ�ϲ����Ըߵ�ѹǿ(5Mpa)��250�棬����Ϊ�������£�ԭ����ת�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com