
| Ӧ��ȡ��Ũ��������/mL | Ӧѡ�õ�����ƿ�Ĺ��/mL | ������ƿ����Ҫ���������� |
���� ��1������������Һ���ѡ������ƿ���������Һϡ���������ʵ����ʵ������������ҪŨ�����������������һ�����ʵ���Ũ��һ�㲽��ѡ����Ҫ������
��2������һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ���ݴ�����
��3������C=$\frac{n}{V}$�����жϣ�
��4��E�м�����ˮ���������˿̶��ߣ�������Һ���ƫ������C=$\frac{n}{V}$������������ʵ��ʧ�ܲ��ܲ��ȣ���Ҫ�������ƣ�
��� �⣺��1�����ܶ�1.18g/mL����������Ϊ36.5%��Ũ�������ʵ���Ũ��C=$\frac{1000��1.18��36.5%}{36.5}$=11.8mol/L������500mL 0.1mol/L �����ᣬӦѡ��500mL�������ƿ������ҪŨ�������ΪV��������Һϡ���������ʵ����ʵ��������11.8mol/L��V=500mL��0.1mol/L�����V=4.2mL��
����һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ�����������Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ����Ի���Ҫ����������Ͳ���ձ�������������ͷ�ιܣ�
�ʴ�Ϊ��
| 4.2 | 500 | ��Ͳ���ձ�������������ͷ�ι� |
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע������ƿ���ѡ����Ŀ�ѶȲ���


 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 200.00mL��Һ������ɫ��$��_{ͨ������SO_{2}��}^{��2����ԭ}$CuCl����ɫ������
200.00mL��Һ������ɫ��$��_{ͨ������SO_{2}��}^{��2����ԭ}$CuCl����ɫ������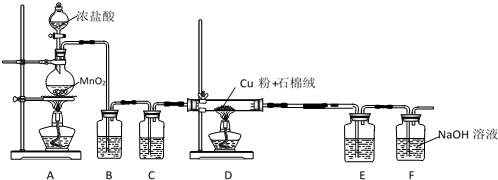
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| Ca2+ | Mg2+ | Fe3+ | |
| ��ʼ������pH | 11.9 | 9.1 | 1.9 |
| ��ȫ������pH | 13.9 | 11.1 | 3.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N2H4��g��+2O2��g���TN2��g��+2H2O��g������H=+534 kJ•L-1 | |
| B�� | N2H4��g��+2O2��g���TN2��g��+2H2O��g������H=-53.4 kJ•L-1 | |
| C�� | N2H4��g��+2O2��g���TN2��g��+2H2O��g������H=+53.4 kJ•L-1 | |
| D�� | N2H4��g��+2O2��g���TN2��g��+2H2O��g������H=-534 kJ•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3��2 | B�� | 2��5 | C�� | 3��4 | D�� | 4��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��OH-��NO3-��HCO3- | B�� | Na+��Fe3+��Cl-��SCN- | ||
| C�� | Na+��Al3+��NO3-��Cl- | D�� | K+��Cu2+��SO42-��OH- |
�鿴�𰸺ͽ���>>
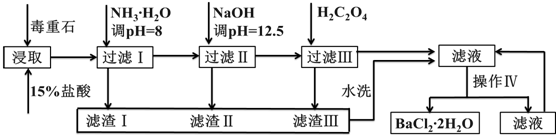
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
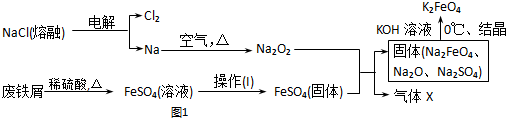
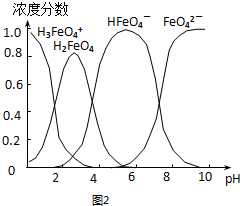
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com