
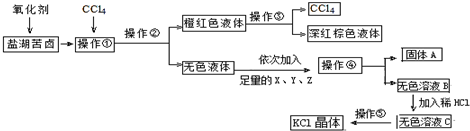
·ÖĪö ×īæŖŹ¼¼ÓµÄŃõ»Æ¼Į½«Br-Ńõ»ÆÉś³ÉBr2£¬ÓɼÓČėCCl4æÉÖŖ£¬ŹĒÓĆŻĶČ”¼ĮŻĶČ”Br2£¬ĖłŅŌ²Ł×÷¢ŁŹĒŻĶČ”£»²Ł×÷¢Ś½«CCl4²ćŗĶĖ®²ć·ÖæŖµÄ¹ż³Ģ½Š×ö·ÖŅŗ£»ĄūÓĆÕōĮó£Ø²Ł×÷¢Ū£©“ÓäåµÄCCl4ČÜŅŗÖŠ·ÖĄėµĆµ½CCl4ŗĶŅŗä壻
²Ł×÷¢ŚµĆµ½µÄĪŽÉ«ČÜŅŗ£¬³żµōŌÓÖŹSO42-£»½«µĆµ½µÄĪŽÉ«ČÜŅŗC½ųŠŠÕō·¢½į¾§µĆµ½KCl¾§Ģ壮
½ā“š ½ā£ŗ£Ø1£©ÓÉĮ÷³ĢæÉÖŖ£¬×īæŖŹ¼¼ÓµÄŃõ»Æ¼Į½«Br-Ńõ»ÆÉś³ÉBr2£¬ÓɼÓČėCCl4æÉÖŖ£¬ŹĒÓĆŻĶČ”¼ĮŻĶČ”Br2£¬ĖłŅŌ²Ł×÷¢ŁŹĒŻĶČ”£»½«CCl4²ćŗĶĖ®²ć·ÖæŖµÄ¹ż³Ģ½Š×ö·ÖŅŗ£¬Ōņ²Ł×÷¢ŚĪŖ·ÖŅŗ£»²Ł×÷¢Ż½«µĆµ½µÄĪŽÉ«ČÜŅŗC½ųŠŠÕō·¢½į¾§µĆµ½KCl¾§Ģ壻
¹Ź“š°øĪŖ£ŗŻĶČ”£»·ÖŅŗ£» Õō·¢½į¾§£»
£Ø2£©²Ł×÷¢ŚĪŖ·ÖŅŗ£¬ŠčŅŖÓƵ½·ÖŅŗĀ©¶·£»
¹Ź“š°øĪŖ£ŗ·ÖŅŗĀ©¶·£»
£Ø3£©BaCl2ČÜŅŗ³żČ„SO42-£»KOHČÜŅŗ³żČ„Mg2+£»ŌŁ¼ÓČėK2CO3ČÜŅŗ£¬³żČ„¹żĮæµÄBaCl2£»
¹Ź“š°øĪŖ£ŗK2CO3£»
£Ø4£©¼ģŃéĪŽÉ«ČÜŅŗBÖŠŹĒ·ńŗ¬ÓŠSO42-·½·ØŹĒ£ŗȔɣĮæČÜŅŗBÓŚŹŌ¹ÜÖŠ£¬ĻņŹŌ¹ÜÄŚµĪ¼ÓBaCl2ČÜŅŗŗĶĻ”ŃĪĖį£¬ČōĪŽ°×É«³Įµķ²śÉśŌņĪŽSO42-£»ČōÓŠ°×É«³Įµķ²śÉśŌņŗ¬ÓŠSO42-£»
¹Ź“š°øĪŖ£ŗȔɣĮæČÜŅŗBÓŚŹŌ¹ÜÖŠ£¬ĻņŹŌ¹ÜÄŚµĪ¼ÓBaCl2ČÜŅŗŗĶĻ”ŃĪĖį£¬ČōĪŽ°×É«³Įµķ²śÉśŌņĪŽSO42-£»ČōÓŠ°×É«³Įµķ²śÉśŌņŗ¬ÓŠSO42-£®
µćĘĄ ±¾ĢāŅŌäå¼°Ęä»ÆŗĻĪļæ¼²é»ģŗĻĪļ·ÖĄėĢį“棬°ŃĪÕĮ÷³ĢÖŠµÄ·ÖĄė·½·ØĪŖ½ā“šµÄ¹Ų¼ü£¬×¢Ņā³£¼ūĪļÖŹµÄŠŌÖŹ¼°»ģŗĻĪļµÄ·ÖĄė·½·Ø¼“æɽā“š£¬ĢāÄæÄѶČÖŠµČ£®


 ¶į¹ŚŃµĮ·µ„ŌŖĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
¶į¹ŚŃµĮ·µ„ŌŖĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø ŠĀĖ¼Ī¬Š”¹Ś¾ü100·Ö×÷Ņµ±¾ĻµĮŠ“š°ø
ŠĀĖ¼Ī¬Š”¹Ś¾ü100·Ö×÷Ņµ±¾ĻµĮŠ“š°ø ĆūŹ¦Öøµ¼Ņ»¾ķĶØĻµĮŠ“š°ø
ĆūŹ¦Öøµ¼Ņ»¾ķĶØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ŗ¬4molHClµÄÅØŃĪĖįÓė×ćĮæMnO2³ä·Ö·“Ó¦£¬×ŖŅĘ2NAøöµē×Ó | |
| B£® | 500”ę”¢30MPaĻĀ£¬½«0.2mol N2ŗĶ0.6molH2ÖĆÓŚĆܱյÄČŻĘ÷ÖŠ³ä·Ö·“Ӧɜ³ÉNH3£Øg£©£¬·ÅČČ7.72kJ£¬ĘäČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ N2£Øg£©+3H2£Øg£© $?_{500”ę”¢30MPa}^{“߻ƼĮ}$ 2NH3£Øg£©”÷H=-38.6kJ•mol-1 | |
| C£® | ¶ŌÓŚæÉÄę·“Ó¦N2£Øg£©+3H2£Øg£©$?_{øßĪĀøßŃ¹}^{“߻ƼĮ}$2NH3£Øg£©£¬”÷H£¼O£»ÉżøßĪĀ¶Č£¬æÉŹ¹·“Ó¦ĖŁĀŹŌö“󣬷“Ó¦ÄęĻņŅĘ¶Æ | |
| D£® | ŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżµÄ¶ąÉŁÓėĘä·Ē½šŹōŠŌµÄĒæČõĪŽ±ŲČ»ĮŖĻµ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

 £¬øĆ·“Ó¦ŹōÓŚČ”“ś·“Ó¦£¬ÓŠ»śĪļµÄĆū³ĘŹĒĻõ»ł±½£®
£¬øĆ·“Ó¦ŹōÓŚČ”“ś·“Ó¦£¬ÓŠ»śĪļµÄĆū³ĘŹĒĻõ»ł±½£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
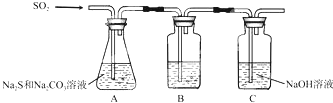
| ŠņŗÅ | ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻó | ½įĀŪ |
| ¢Ł | ȔɣĮæѳʷӌŹŌ¹ÜÖŠ£¬¼ÓČėŹŹĮæÕōĮóĖ®£¬³ä·ÖÕńµ“Čܽā µĪ¼Ó×ćĮæĻ”ĻõĖį£¬ŌŁµĪ¼ÓÉŁĮæAgNO3ČÜŅŗ£¬Õńµ“ | ÓŠ°×É«³ĮµķÉś³É | ѳʷŗ¬NaCl |
| ¢Ś | ȔɣĮæѳʷӌŹŌ¹ÜÖŠ£¬¼ÓČėŹŹĮæÕōĮóĖ®£¬³ä·ÖÕńµ“Čܽā ¼ÓČė¹żĮæBaCl2ČÜŅŗ£¬½Į°č£¬¾²ÖĆ£¬ÓĆpH¼Ę²ā¶ØÉĻ²ćĒåŅŗpH | ÓŠ°×É«³ĮµķÉś³É£¬ÉĻ²ćĒåŅŗpH“óÓŚ9.6 | ѳʷŗ¬NaOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | MgCl2 | B£® | CO2 | C£® | KOH | D£® | ½šøÕŹÆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 5730”Į3Äź | B£® | 5730”Į4Äź | C£® | 5730”Į6Äź | D£® | 5730”Į8Äź |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
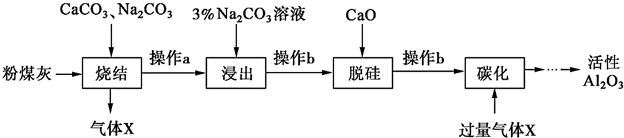
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
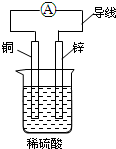
| A£® | ŠæʬĪŖøŗ¼«£¬ĶʬĪŖÕż¼« | |
| B£® | øĆ×°ÖĆÄܽ«µēÄÜ×Ŗ»ÆĪŖ»ÆѧÄÜ | |
| C£® | Ķʬ·¢ÉśµÄ·“Ó¦ĪŖCu-2e-ØTCu2+ | |
| D£® | µēĮ÷·½ĻņŹĒÓÉŠæʬĶعżµ¼ĻßĮ÷ĻņĶʬ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com