
 ��
������ ��1������֪ˮú������Ҫ�ɷ֣�CO��H2��ÿ�ϳ�1g�״����ͷų�akJ��������������1mol�״�����Ϊ32g�����ͷ�32aKJ��������ע��״�ΪҺ�壻
������3CH3OH+NH3�TC3H7N+3H2O����֪�÷�ӦΪȡ����Ӧ��������������ԭ�ӱ�������ȡ�������ݰ����ĵ���ʽ������д���װ��ĵ���ʽ��
��2�������������Ϊ�������ʽ�����ӣ��ȴ���ˮ�⣬Ҳ���ڵ��룬ˮ����ڵ�������Һ�Լ��ԣ��������ˮ���������ԣ����������ˮ�ⷽ��ʽ��HS-+H2O?H2S+OH-��ͨ����������������������ӷ�Ӧ�������ʣ�������������ӣ�
����֪̼�������ǿ�������ᣬ����������Խ��Խˮ��Ĺ��ɿ�֪��������ˮ������ǿ��̼������ӣ�̼�������Ũ�ȴ���������Ũ�ȣ�̼�������ˮ��ˮ�����ɵ����ʵ�����̼��������������������ӣ�������ˮ�����ɵ����ʵ�������������Ӻ����������ӣ�������ˮ��̶ȴ���̼������ӣ��������ɵ����������Ӵ�����������ӣ����������Ũ�ȴ���̼���������Ũ�ȣ���Һ����ˮ��̼��������ӡ���������ӵ���������������ӣ�����������Ũ����С��
������������ͭ��Ӧ�����������ͭ���������������������C��Cu2+����C��S2-���ܶȻ������ж��Ƿ��ܲ���������
��� �⣺��1������֪ˮú������Ҫ�ɷ֣�CO��H2��ÿ�ϳ�1g�״����ͷų�akJ��������������1mol�״�����Ϊ32g�����ͷ�32aKJ��������ע��״�ΪҺ�壬���Ȼ�ѧ����ʽ��2H2��g��+CO��g��=2CH3OH��l����H=-32akJ/mol��
�ʴ�Ϊ��2H2��g��+CO��g��=2CH3OH��l����H=-32akJ/mol��
�ڰ�����������ԭ�ӱ�������ȡ�������ݰ����ĵ���ʽ��֪���װ��ĵ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��2�������������Ϊ�������ʽ�����ӣ��ȴ���ˮ�⣬Ҳ���ڵ��룬ˮ���������⣬�������������ӣ���֪NaHS��Һ��S2-��Ũ��С��H2S��Ũ�ȣ�˵��ˮ��С�ڵ��룬��Һ�Լ��ԣ����������ˮ�ⷽ��ʽ��HS-+H2O?H2S+OH-��ͨ����������������������ӷ�Ӧ�������ʣ�������������ӣ�����Һ��$\frac{c��{H}_{2}S��}{c��H{S}^{-}��}$�����
�ʴ�Ϊ�����ԣ����
����֪̼�������ǿ�������ᣬ����������Խ��Խˮ��Ĺ��ɿ�֪��������ˮ������ǿ��̼������ӣ�̼�������Ũ�ȴ���������Ũ�ȣ�̼�������ˮ��ˮ�����ɵ����ʵ�����̼��������������������ӣ�������ˮ�����ɵ����ʵ�������������Ӻ����������ӣ�������ˮ��̶ȴ���̼������ӣ��������ɵ����������Ӵ�����������ӣ����������Ũ�ȴ���̼���������Ũ�ȣ���Һ����ˮ��̼��������ӡ���������ӵ���������������ӣ�����������Ũ����С��������Һ����Ũ�ȴӴ�С˳��Ϊ��c��Na+����c��CO32-����c��S2-����c��OH-����c��HCO3-��c��HS-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��S2-����c��OH-����c��HCO3-��c��HS-����c��H+����
�۽���������ͨ������ͭ��Һ�����ӷ���ʽ��Cu2++H2S�TCuS��+2H+��
����Ϊ��Ԫ���ᣬ�ֲ����룬���������֪Ka1=9.1��10-8��Ka2=1.1��10-23��
Ka1=$\frac{C��H{\;}^{+}��C��HS{\;}^{-}��}{C��{H}_{2}S��}$��Ka2=$\frac{C��H{\;}^{+}��C��{S}^{2}{\;}^{-}��}{C��HS{\;}^{-}��}$����C��S2-��=$\frac{K{a}_{1}��K{a}_{2}��C��{H}_{2}S��}{{C}^{2}��H{\;}^{+}��}$=$\frac{9.1��1{0}^{-8}��1.1��1{0}^{-23}��2��1{0}^{-3}}{��10{\;}^{-4}��{\;}^{2}}$=2.02��10-25��
Qc=C��S2-��C��Cu2+��=2.02��10-25��2��10-4��=4.04��10-29��
��Qc��Ksp�����������������
�ʴ�Ϊ��Cu2++H2S�TCuS��+2H+���У�Qc��Ksp��
���� ����Ϊ�ۺ��⣬�������Ȼ�ѧ����ʽ����д������ʽ����д������Ũ�ȴ�С�Ƚϡ��ܶȻ������Ӧ�ã�ע����д�Ȼ�ѧ����ʽӦע����Ӧ��ۼ�״̬����Ӧ�����������������ȣ�ע������ˮ����ɡ��ܶȻ������Ӧ�ã���Ŀ�ѶȽϴ��ؿ���ѧ��������֪ʶ��Ǩ����Ӧ���������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��NH3������Nԭ�ӵ��ӻ���ʽΪSP3�ӻ���NH3���ӵĿռ����幹���������ͣ�
��NH3������Nԭ�ӵ��ӻ���ʽΪSP3�ӻ���NH3���ӵĿռ����幹���������ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
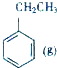 +CO2��g��?
+CO2��g��?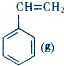 +CO��g��+H2O��g����H
+CO��g��+H2O��g����H ?
? +H2��g����H1=+117.6kJ/mol
+H2��g����H1=+117.6kJ/mol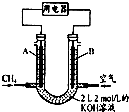
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ̼��������Ȼ���й㷺���ڣ�
̼��������Ȼ���й㷺���ڣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������������к�������Ԫ�� | |
| B�� | ����������ʵ�ļ����Ԫ�� | |
| C�� | ����������Ⱦ��������Դ | |
| D�� | ��̬�������⡱�����Ĺ������������仯 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com