
 ��ˮ�еĵ��뷽��ʽΪ
��ˮ�еĵ��뷽��ʽΪ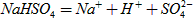 ����
���� ��Һ
��Һ
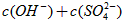 ���>����=����<������
���>����=����<������ 
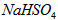 ��Һ����μ�������ʵ���Ũ�ȵ�
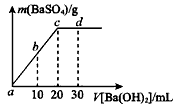
��Һ����μ�������ʵ���Ũ�ȵ�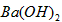 ��Һ�����������������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ��ʱ��ͬ�ε���Һ������b���ʾ��Һ��_________������ԡ������ԡ����ԡ�����c����ʾ�����ӷ���ʽΪ___________________��
��Һ�����������������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ��ʱ��ͬ�ε���Һ������b���ʾ��Һ��_________������ԡ������ԡ����ԡ�����c����ʾ�����ӷ���ʽΪ___________________��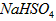 ������뵽pH=6������ˮ�У������¶Ȳ��䣬�����Һ��pHΪ2��T�潫
������뵽pH=6������ˮ�У������¶Ȳ��䣬�����Һ��pHΪ2��T�潫 Ϊ
Ϊ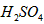 ��ҺV2L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH=2����V1: V2 =____________������Һ�и������ӵ�Ũ���ɴ�С������˳��Ϊ_________________________��
��ҺV2L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH=2����V1: V2 =____________������Һ�и������ӵ�Ũ���ɴ�С������˳��Ϊ_________________________��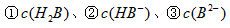 �ɴ�С��˳��Ϊ
�ɴ�С��˳��Ϊ ��Һ����
��Һ����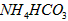 ��Һ����
��Һ���� ��Һ�У�
��Һ�У�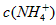 �ɴ�С������˳��Ϊ_____________________��
�ɴ�С������˳��Ϊ_____________________��  �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ش����и�С�⣺
�ش����и�С�⣺| O | 2- 4 |
| O | 2- 4 |
| H | + 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧʽ | ���볣�� |
| HClO | Ka=3��10-8 |
| H2CO3 | Ka1=4.3��10-7 |
| Ka2=5.6��10-11 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и�����ѧ����ĩģ�⻯ѧ�Ծ��������棩 ���ͣ������
��10�֣��������ԭ�����ش����и�С�⣺
��1��25��ʱ��ijFeCl3��Һ��pH=2������ˮ�����������c(OH-)= �������ӷ���ʽ��ʾFeCl3��Һ���ھ�ˮ��ԭ�� ��
��2����֪NaHSO4��ˮ�еĵ��뷽��ʽNaHSO4��Na+��H+��SO42-��
��NaHSO4��Һ��c(H+) c(OH-)��c(SO42-)���>������������<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ��������Ӧ����Һ��pH 7��
��3����0.02mol/LNa2SO4��Һ��ijŨ��BaCl2��Һ�������ϣ�������BaSO4��������ԭBaCl2��Һ����СŨ��Ϊ ��(��֪Ksp(BaSO4)��1.1��10-10)
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
����������Ӧ �� �Ҵ��Ĵ�������Ӧ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com