
�����йص���������ȫ��ȷ��һ����
��ֱ�Ӽ��������Һ����ijʳ���Ƿ�Ϊ�ӵ��Σ��ڳ��³�ѹ�£�14g��C3H6��C4H8��������к��е�ԭ����Ϊ3NA������ͨ��״���£�0.5molNO��0.5molO2��Ϻ����������Ϊ0.75 NA����������Ϊ���Ե缫�����ȷŵ磬��Cu��Fe��Al��Ag����������Ϊ���Ե缫����˶��ǵ缫����ʧ���ӣ���1 molC10H22�����к��еĹ��ۼ�������Ϊ31 NA���ޱ�״���£�22.4LSO3�к��еķ�����ΪNA�������ϴ�����ֽ��ë��������Ʒ�Ⱦ������л������������ﶼ���ɽ���Ԫ�غ���Ԫ����ɣ���K2O��CuO��Na2O��Na2O2��Mn2O7��Al2O3��Fe2O3��ȫ���ɽ���Ԫ�غ���Ԫ����ɵģ���˶�Ϊ����������
A���٢ڢۢݢ� B���٢ۢޢߢ� C���٢ܢޢ� D���ڢܢݢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A������Ӧ��Ũ�ȣ����������ӵİٷ������Ӷ�ʹ��Ч��ײ��������
B��������μӵĻ�ѧ��Ӧ��������ѹǿ������С��Ӧ������������������ӻ���ӵİٷ������Ӷ�ʹ��Ӧ��������
C�������¶���ʹ��ѧ��Ӧ����������Ҫԭ���������˷�Ӧ������л���ӵİٷ���
D��������Ӱ�췴Ӧ��ܵ�������λ����ڻ���Ӱٷ������Ӷ�����Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧʵ��װ�õ���ȷ������ʵ��ɹ��Ĺؼ�����ͼ����ѧ��ѧ�г�����ʵ��װ�á�
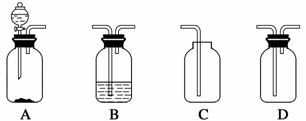
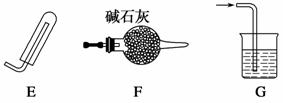
(1)������װ���Ʊ����壺
����B��ʢ��Ũ���ᣬ��A��B��C��Ϻ�����ȡ���ռ���������________��
a��H2 b��H2S����
c��CO2 d��C2H2
��Ҫ������ȡ���õ������NH3����ȷ�����������____(������װ��˳���������ı����ĸ)��������ѡ�õĹ���ҩƷ��__________��
������H2O2��MnO2����ȡ���ռ������O2����Ӧѡ���������ȷ�����__________(������װ��˳���������ı����ĸ)�����������ռ����ķ�����________________��
(2)��ͬѧ��A��B�������֤���ᡢ̼�ᡢ�����������ǿ��ʱ������Ӧװ��__________(����������)�У�Bװ���з�����Ӧ�����ӷ���ʽΪ_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ��ͨ��һ����SO2������ֳ�������(����)
A��Na2S��Һ B��BaCl2��Һ
C��Ba(NO3)2��Һ D��NaClO��BaCl2�����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�����Һ,����ֻ����Fe2+��Cl-��Br-��I-������ˮ�ĵ��룩������Cl-��Br-��I-�ĸ�����Ϊ2��3��4�������Һ��ͨ��������ʹ��Һ��Cl-��Br-�ĸ�����Ϊ3��1����ͨ�����������ʵ�������Һ��ʣ���Fe2+�����ʵ���֮��Ϊ����ԭ��I����Fe2+��Br-��Cl-��(����)
A��7��1 B��7��2 C��7��3 D��7��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij��Һ�п��ܺ���NH4+��SO32—��SO42����NO3��������ijЩδ֪���ӣ�ijѧ��ȡ������Һ�����Թ��У�Ȼ���������ʵ�鲢�ó���Ӧ�Ľ��ۣ����к�������
A������BaCl2��Һ�õ���ɫ�����˲�ϴ�ӳ�����Ȼ�������뵽������ϡ�����У��������κα仯��˵������Һ��һ������SO42��
B������BaCl2��Һ�õ���ɫ�����˲�ϴ�ӳ�����Ȼ�������뵽������ϡ�����У������ܽⲢ�����̼�����ζ�����壬˵������Һ��һ������SO32��
C������1mL0.2mol/L��NaOH����ʪ�����ɫʯ����ֽ�����Թܿڣ���ֽ�ޱ仯��˵����Һ��һ����NH4+
D������пƬ���ޱ仯���ټ���������ϡ���ᣬпƬ�ܽⲢ�����ݲ���������Һ��һ������NO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ļ�ѧʽΪC2H4O�����ڻ������������˵����ȷ����
A������������ B�����Ǻ���3��Ԫ�صĻ����
C������Ħ��������44g D�����£�1 mol�������麬��2NA��̼ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ90 mLŨ��Ϊ1 mol·L��1��ϡ���ᣬ����18.4 mol·L��1��Ũ�������ƣ�������ɷ�Ϊ���¸�����
A������Ͳ��ȡ5.4mLŨ���Ỻ��ע��װ��Լ10 mL����ˮ���ձ��У����ò��������Ͻ������____________��
B����Լ20 mL����ˮ��������ϴ���ձ��Ͳ���������ÿ��ϴҺ����������ƿ�
C����ϡ�ͺ�ϡ����С�ĵ���____________mL����ƿ�
D�������ѡ����ƿ�Ƿ�ᷢ��©Һ��
E��������ˮֱ�Ӽ�������ƿ����Һ�����̶���________________����
F���ǽ�ƿ���������ߵ���ҡ����Һ��
G���ý�ͷ�ι�������ƿ����ε�������ˮ��Ŀ��ƽ�ӣ�����_____________��
(1)��д���������Ŀհ״���
(2)��ȷ�IJ���˳����D A C _____________ F (��д��ĸ)��
(3)����A������Ӧѡ��������������10 mL��Ͳ����50 mL��Ͳ��500 mL��Ͳ����1 000 mL��Ͳ�е�______(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йص������Һ��˵����ȷ���ǣ� ��
A����Ca��ClO��2��Na2SO3��FeCl3��Һ���ɾ��ò���ԭ����
B�������Ȼ�������Һʱ������Һ�з��������ۣ��Է�ֹFe2+ˮ��
C�������£���0.1mol/L��CH3COOH��Һ�м�������ˮ��Һ�Լ��Ե����ʣ�CH3COOH�ĵ���̶�һ������
D��NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com