
X”¢Y”¢Z”¢WĪŖŗ¬ÓŠĻąĶ¬µē×ÓŹżµÄ·Ö×Ó»ņĄė×Ó£¬¾łÓÉŌ×ÓŠņŹżŠ”ÓŚ10µÄŌŖĖŲ×é³É£¬XÓŠ5øöŌ×ÓŗĖ”£Ķس£×“æöĻĀ£¬WĪŖĪŽÉ«ŅŗĢ唣
ŅŃÖŖ:X+Y Z+W
Z+W
(1)YµÄµē×ÓŹ½ŹĒ_________________________”£
(2)ŅŗĢ¬ZŗĶWµÄµēĄėĻąĖĘ£¬¶¼æɵēĄė³öµē×ÓŹżĻąĶ¬µÄĮ½ÖÖĄė×Ó£¬ŅŗĢ¬ZµÄµēĄė·½³ĢŹ½ŹĒ_________________________________”£
(3)ÓĆĶ¼Ź¾×°ÖĆÖʱøNO²¢ŃéÖ¤Ę仹ŌŠŌ”£ÓŠĻĀĮŠÖ÷ŅŖ²Ł×÷£ŗ
a.Ļņ¹ćæŚĘæÄŚ×¢Čė×ćĮæČČNaOHČÜŅŗ£¬½«Ź¢ÓŠĶʬµÄŠ”ÉÕ±·ÅČėĘæÖŠ”£
b.Ö¹Ė®¼Š£¬µćČ¼ŗģĮ×£¬ÉģČėĘæÖŠ£¬ČūŗĆ½ŗČū”£
c.“żŗģĮ׳ä·ÖČ¼ÉÕ£¬Ņ»¶ĪŹ±¼äŗó“ņæŖ·ÖŅŗĀ©¶·ŠżČū£¬ĻņÉÕ±ÖŠµĪČėÉŁĮæĻ”ĻõĖį”£

¢Ł²½Öčcŗó»¹Č±ÉŁµÄŅ»²½Ö÷ŅŖ²Ł×÷ŹĒ_______________________________________”£
¢ŚŗģĮ׳ä·ÖČ¼ÉյIJśĪļÓėNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ______________________________________”£
¢Ū²½ÖčcµĪČėĻ”ĻõĖįŗóÉÕ±ÖŠµÄĻÖĻóŹĒ______________________________________
____________________________________________________________________ӣ
·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ____________________________________________________”£
(4)Ņ»¶ØĪĀ¶ČĻĀ£¬½«1 mol N2O4ÖĆÓŚĆܱÕČŻĘ÷ÖŠ£¬±£³ÖŃ¹Ēæ²»±ä£¬ÉżøßĪĀ¶ČÖĮT1µÄ¹ż³ĢÖŠ£¬ĘųĢåÓÉĪŽÉ«Öš½„±äĪŖŗģ×ŲÉ«”£ĪĀ¶ČÓÉT1¼ĢŠųÉżøßµ½T2µÄ¹ż³ĢÖŠ£¬ĘųĢåÖš½„±äĪŖĪŽÉ«”£Čō±£³ÖT2£¬Ōö“óŃ¹Ē棬ĘųĢåÖš½„±äĪŖŗģ×ŲÉ«”£ĘųĢåµÄĪļÖŹµÄĮænĖęĪĀ¶ČT±ä»ÆµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£

¢ŁĪĀ¶ČŌŚT1”«T2Ö®¼ä£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_________________________”£
¢ŚĪĀ¶ČŌŚT2”«T3Ö®¼ä£¬ĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæŹĒ£Ø±£Įō1Ī»Š”Źż£©______________”£
”¾“š°ø”æ
£Ø1£©
£Ø2£©2NH3(I)
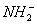 +
+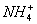
(3)¢Ł“ņæŖÖ¹Ė®¼Š£¬ĶØČėÉŁĮæŃõĘų
¢ŚP2O5+6OH- 2
2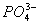 +3H2O
+3H2O
¢ŪCuʬ֚½„Čܽā£¬ÓŠĪŽÉ«ĘųÅŻ²śÉś£¬ČÜŅŗÓÉĪŽÉ«±äĪŖĄ¶É«
3Cu+8H++2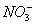
 3Cu2++2NOӟ+4H2O
3Cu2++2NOӟ+4H2O
(4)¢Ł2NO2  2NO+O2
2NO+O2
¢Ś30.7
”¾½āĪö”æøł¾ŻĢāŅŃÖŖĢõ¼žæÉÖŖ£¬XĪŖ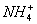 £¬WĪŖH2O£¬ÓÉX+Y
£¬WĪŖH2O£¬ÓÉX+Y Z+WæÉÖŖYĪŖOH-£¬ZĪŖNH3£¬¼“
Z+WæÉÖŖYĪŖOH-£¬ZĪŖNH3£¬¼“
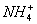 +OH-
+OH- NH3+H2O
NH3+H2O
(2)Ņŗ°±£ØNH3£©ÓėW(H2O)µēĄėĻąĖĘ£¬²ĪÕÕĖ®µÄµēĄė£ŗ2H2O H3O++OH-£¬Ņŗ°±µÄµēĄė·½³ĢŹ½ĪŖ£ŗ2NH3(l)
H3O++OH-£¬Ņŗ°±µÄµēĄė·½³ĢŹ½ĪŖ£ŗ2NH3(l)
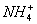 +
+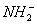 ӣ
ӣ
(3)¢ŁÓÉÓŚ±¾ŹµŃéÄæµÄŹĒ”°ÖʱøNO²¢ŃéÖ¤Ę仹ŌŠŌ”±£¬Ņņ“ĖŌŚ²½ÖčCÖʵĆNOŗó»¹Č±ÉŁµÄŅ»²½Ö÷ŅŖ²Ł×÷ŹĒ£ŗ“ņæŖÖ¹Ė®¼Š£¬·Å½ųŅ»²æ·ÖŃõĘų£¬ŅŌŃéÖ¤NOµÄ»¹ŌŠŌ”£
¢ŚŗģĮ׳ä·ÖČ¼ÉյIJśĪļĪŖP2O5£¬
P2O5+6NaOH 2Na3PO4+3H2O£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗ
2Na3PO4+3H2O£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗ
P2O5+6OH- 2
2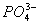 +3H2O
+3H2O
¢ŪĻņÉÕ±ÖŠµĪČėĻ”HNO3ŗó·¢ÉśµÄĻÖĻóĪŖ£ŗĶʬĀżĀżČܽā£¬ÓŠĪŽÉ«ĘųÅŻ²śÉś£¬ČÜŅŗ±ä³ÉĄ¶É«£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ
3Cu+2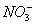 +8H+
+8H+ 3Cu2++2NOӟ+4H2O
3Cu2++2NOӟ+4H2O
(4)¢ŁĪĀ¶ČÓÉT1Éżøßµ½T2£¬ĘųĢåµÄĪļÖŹµÄĮæŌö¼Ó²¢±äĪŖĪŽÉ«£¬ĖµĆ÷NO2·¢ÉśĮĖ·Ö½ā·“Ó¦£¬Éś³ÉĪŽÉ«ĘųĢ壬½įŗĻÖŹĮæŹŲŗć¶ØĀÉæÉÖŖ·“Ó¦·½³ĢŹ½ĪŖ£ŗ
2NO2(g) 2NO(g)+O2(g)ӣ
2NO(g)+O2(g)ӣ
¢Śøł¾Ż·“Ó¦¹ż³ĢÖŠÖŹĮæŹŲŗć£¬ŌņT2”ŖT3Ź±»ģŗĻĘųĢåĻą¶Ō·Ö×ÓÖŹĮæĪŖ£ŗ =30.7 g”¤mol-1£¬¼“Ļą¶Ō·Ö×ÓÖŹĮæĪŖ30.7”£
=30.7 g”¤mol-1£¬¼“Ļą¶Ō·Ö×ÓÖŹĮæĪŖ30.7”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹĒŅ»ÖÖ¶ŌøŹÕįŌü½ųŠŠ·ĻĪļĄūÓƵÄÉčĻė£ŗ
£Ø1£©ŅŃÖŖAÄÜÓėNaHCO3ČÜŅŗ·“Ó¦²śÉśĘųÅŻ£¬1molAŗĶ×ćĮæµÄ½šŹōÄĘ·“Ó¦²śÉś22.4L£Ø±ź×¼×“æöĻĀ£©H2£¬ŌņA·Ö×ÓÖŠŗ¬ÓŠµÄŗ¬Ńõ¹ŁÄÜĶŵÄĆū³ĘŹĒ £®
£Ø2£©ŅŌAĪŖŌĮĻæÉŅŌÖĘČ”Ņ»ÖÖ³£¼ūŹ÷Ö¬M£ŗ
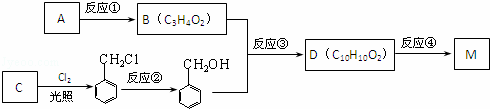
¢ŁC”¢MµÄ½į¹¹¼ņŹ½·Ö±šĪŖ£ŗC M £»
¢Ś·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶĪŖ £»
¢Ū·“Ó¦¢ŚĖł¼ÓµÄŹŌ¼ĮŹĒ £»
¢Ü·“Ó¦¢ŪµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ £»
¢ŻDÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬ĘäÖŠÓŠŅ»Ąą·ūŗĻĻĀĮŠĢõ¼ž£ŗ
£Øa£©±½»·ÉĻÓŠ2øöČ”“ś»łĒŅ“¦ÓŚ¶ŌĪ»
£Øb£©ÄÜŹ¹FeCl3ČÜŅŗĻŌÉ«
£Øc£©ÄÜ·¢ÉśŅų¾µ·“Ó¦
ĒėŠ“³ö·ūŗĻÉĻŹöĢõ¼žµÄŅ»ÖÖÓŠ»śĪļµÄ½į¹¹¼ņŹ½£ŗ µČ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«22.4 LijĘųĢ¬µŖŃõ»ÆŗĻĪļÓė×ćĮæµÄ×ĘČČĶ·ŪĶźČ«·“Ó¦ŗó£¬ĘųĢåĢå»ż±äĪŖ11.2 L(Ģå»ż¾łŌŚĻąĶ¬Ģõ¼žĻĀ²ā¶Ø)£¬ŌņøƵŖŃõ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ( )
A.NO2 B.N2O3 C.N2O D.N2O4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĘųĢåµÄĦ¶ūÖŹĮæŌ½Š”£¬Ą©É¢ĖŁ¶ČŌ½æģ”£ĻĀĶ¼ĖłŹ¾ĪŖĘųĢåĄ©É¢ĖŁ¶ČµÄŹµŃ飬Į½ÖÖĘųĢåĄ©É¢ĻąÓöŹ±ŠĪ³É°×É«ŃĢ»·”£ĻĀĮŠ¹ŲÓŚĪļÖŹ¼×”¢ŅŅµÄÅŠ¶ĻÕżČ·µÄŹĒ£Ø £©

A.¼×ŹĒÅØ°±Ė®£¬ŅŅŹĒÅØĮņĖį B.¼×ŹĒÅØŃĪĖį£¬ŅŅŹĒÅØ°±Ė®
C.¼×ŹĒÅØ°±Ė®£¬ŅŅŹĒÅØŃĪĖį D.¼×ŹĒÅØĻõĖį£¬ŅŅŹĒÅØ°±Ė®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŹÆ»ŅŅ¤ÖŠÉÕÖĘÉśŹÆ»Ņ£¬1 mol CaCO3ĶźČ«·Ö½āĖłŠčŅŖµÄÄÜĮ棬æÉČ¼ÉÕ0.453 molĢ¼Ą“Ģį¹©”£ÉčæÕĘųÖŠO2Ģå»ż·ÖŹżĪŖ0.21£¬N2ĪŖ0.79£¬ŌņŹÆ»ŅŅ¤²śÉśµÄĘųĢåÖŠCO2µÄĢå»ż·ÖŹżæÉÄÜŹĒ£Ø £©
A.0.43 B.0.46 C.0.49 D..0.52
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
A.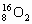 ŗĶ
ŗĶ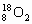 »„ĪŖĶ¬Ī»ĖŲ£¬ŠŌÖŹĻąĖĘ
»„ĪŖĶ¬Ī»ĖŲ£¬ŠŌÖŹĻąĖĘ
B.³£ĪĀĻĀ£¬pH=1µÄĖ®ČÜŅŗÖŠNa+”¢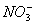 ”¢
”¢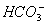 ”¢Fe2+æÉŅŌ“óĮæ¹²“ę
”¢Fe2+æÉŅŌ“óĮæ¹²“ę
C.Ć÷·ÆŗĶĘÆ°×·Ū³£ÓĆÓŚ×ŌĄ“Ė®µÄ¾»»ÆŗĶɱ¾śĻū¶¾£¬Į½ÕßµÄ×÷ÓĆŌĄķĻąĶ¬
D.C(ŹÆÄ«£¬s)=C(½šøÕŹÆ£¬s)”÷H£¾0£¬ĖłŅŌŹÆÄ«±Č½šøÕŹÆĪȶØ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹµŃéĻÖĻóµÄĆčŹö“ķĪóµÄŹĒ
A. ĒāĘųŌŚĀČĘųÖŠČ¼ÉÕÉś³ÉĀĢÉ«ŃĢĪķ
B. ŗģČȵÄĢśĖæŌŚŃõĘųÖŠČ¼ÉÕ£¬»šŠĒĖÄÉä£¬Éś³ÉŗŚÉ«¹ĢĢåæÅĮ£
C. µćČ¼µÄĮņŌŚŃõĘųÖŠ¾ēĮŅČ¼ÉÕ£¬·¢³öĄ¶×ĻÉ«»šŃę
D.ÄĘŌŚæÕĘųÖŠČ¼ÉÕ£¬·¢³ö»ĘÉ«µÄ»šŃę£¬Éś³Éµ»ĘÉ«¹ĢĢå
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻŅŌĮņĢśæóĪŖŌĮĻÖĘĮņĖįĖł²śÉśµÄĪ²Ęų³żĮĖŗ¬ÓŠN2”¢O2Ķā£¬»¹ŗ¬ÓŠSO2”¢Ī¢ĮæµÄSO3ŗĶĖį Īķ”£ĪŖĮĖ±£»¤»·¾³£¬Ķ¬Ź±ĢįøßĮņĖį¹¤ŅµµÄ×ŪŗĻ¾¼ĆŠ§Ņę£¬Ó¦¾”æÉÄܽ«Ī²ĘųÖŠµÄSO2×Ŗ»ÆĪŖÓŠÓƵÄø±²śĘ·”£Ēė°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
Īķ”£ĪŖĮĖ±£»¤»·¾³£¬Ķ¬Ź±ĢįøßĮņĖį¹¤ŅµµÄ×ŪŗĻ¾¼ĆŠ§Ņę£¬Ó¦¾”æÉÄܽ«Ī²ĘųÖŠµÄSO2×Ŗ»ÆĪŖÓŠÓƵÄø±²śĘ·”£Ēė°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©½«Ī²ĘųĶØČė°±Ė®ÖŠ£¬ÄÜ·¢Éś¶ąøö·“Ó¦£¬Š“³öĘäÖŠæÉÄÜ·¢ÉśµÄĮ½øöŃõ»Æ»¹Ō·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”¢ ”£
£Ø2£©ŌŚĪ²ĘųÓė°±Ė®·“Ó¦ĖłµĆµ½µÄøßÅضČČÜŅŗÖŠ£¬°“Ņ»¶Ø±ČĄż¼ÓČė°±Ė®»ņĢ¼ĖįĒāļ§£¬“ĖŹ±ČÜŅŗµÄĪĀ¶Č»į×ŌŠŠ½µµĶ£¬²¢Īö³ö¾§Ģ唣¢Łµ¼ÖĀČÜŅŗĪĀ¶Č½µµĶµÄŌŅņæÉÄÜŹĒ ;¢ŚĪö³öµÄ¾§ĢåæÉÓĆÓŚŌģÖ½¹¤Ņµ£¬Ņ²æÉÓĆÓŚÕÕĻąÓĆĻŌÓ°ŅŗµÄÉś²ś”£ŅŃÖŖøĆ½į¾§Ė®ŗĻĪļµÄĻą¶Ō·Ö×Ó ÖŹĮæĪŖ134£¬ŌņĘä»ÆѧŹ½ĪŖ £»¢ŪÉś²śÖŠĶłĶłŠčŅŖĻņČÜŅŗÖŠ¼ÓČėŹŹĮæµÄ¶Ō±½¶ž·Ó»ņ¶Ō±½¶ž°·µČĪļÖŹ£¬ĘäÄæµÄŹĒ ”£
ÖŹĮæĪŖ134£¬ŌņĘä»ÆѧŹ½ĪŖ £»¢ŪÉś²śÖŠĶłĶłŠčŅŖĻņČÜŅŗÖŠ¼ÓČėŹŹĮæµÄ¶Ō±½¶ž·Ó»ņ¶Ō±½¶ž°·µČĪļÖŹ£¬ĘäÄæµÄŹĒ ”£
£Ø3£©ÄÜÓĆÓŚ²ā¶ØĮņĖįĪ²ĘųÖŠSO2ŗ¬ĮæµÄŹĒ ”££ØĢī×ÖÄø£©
A.NaOHČÜŅŗ”¢·ÓĢŖŹŌŅŗ B.KMnO4ČÜŅŗ”¢Ļ”H2SO4
C.µāĖ®”¢µķ·ŪČÜŅŗ D.°±Ė®”¢·ÓĢŖŹŌŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
| ”” | A£® | 1molČĪŗĪĘųĢåĖłŗ¬·Ö×ÓŹż¶¼ĻąĶ¬£¬Ģå»żŅ²¶¼Ō¼ĪŖ22.4 L |
| ”” | B£® | 1molKClO3ŗĶ1molSO2ÖŠ£¬Ėłŗ¬ŃõŌ×ÓµÄøöŹż±ČĪŖ3£ŗ2 |
| ”” | C£® | ĪļÖŹµÄĮæ¾ĶŹĒĪļÖŹµÄĦ¶ūÖŹĮæ |
| ”” | D£® | ŌŚ·Ē±ź×¼×“æöĻĀ£¬1 molČĪŗĪĪļÖŹµÄĢå»ż¶¼²»ŹĒ22.4 L |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com