
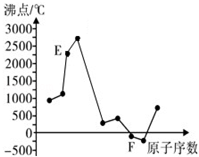
 A”¢B”¢C”¢D”¢E”¢FŹĒŌŖĖŲÖÜĘŚ±ķÖŠĒ°20ŗÅŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®A”¢BæÉ·Ö±šÓėC×é³É³£¼ū»ÆŗĻĪļAC”¢AC2”¢BC”¢BC2£»DŌŖĖŲµÄŃęÉ«·“Ó¦³Ź»ĘÉ«£»E”¢FĮ½ŌŖĖŲµ„ÖŹµÄ·ŠµćÓėŌŖĖŲŌ×ÓŠņŹżµÄ¹ŲĻµČēĶ¼£ØĶ¼ÖŠŌ×ÓŠņŹżĮ¬Šų£©£®
A”¢B”¢C”¢D”¢E”¢FŹĒŌŖĖŲÖÜĘŚ±ķÖŠĒ°20ŗÅŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®A”¢BæÉ·Ö±šÓėC×é³É³£¼ū»ÆŗĻĪļAC”¢AC2”¢BC”¢BC2£»DŌŖĖŲµÄŃęÉ«·“Ó¦³Ź»ĘÉ«£»E”¢FĮ½ŌŖĖŲµ„ÖŹµÄ·ŠµćÓėŌŖĖŲŌ×ÓŠņŹżµÄ¹ŲĻµČēĶ¼£ØĶ¼ÖŠŌ×ÓŠņŹżĮ¬Šų£©£®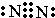 £¬»ÆŗĻĪļAC2µÄ½į¹¹Ź½O=C=O£»C”¢D”¢F¶ŌÓ¦µÄ¼ņµ„Ąė×Ó°ė¾¶ÓÉŠ”µ½“óµÄĖ³ŠņĪŖNa+£¼O2-£¼Cl-£ØÓĆĄė×Ó·ūŗűķŹ¾£©£®
£¬»ÆŗĻĪļAC2µÄ½į¹¹Ź½O=C=O£»C”¢D”¢F¶ŌÓ¦µÄ¼ņµ„Ąė×Ó°ė¾¶ÓÉŠ”µ½“óµÄĖ³ŠņĪŖNa+£¼O2-£¼Cl-£ØÓĆĄė×Ó·ūŗűķŹ¾£©£®·ÖĪö A”¢B”¢C”¢D”¢E”¢FŹĒŌŖĖŲÖÜĘŚ±ķÖŠĒ°20ŗÅŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®DŌŖĖŲµÄŃęÉ«·“Ó¦³Ź»ĘÉ«£¬ŌņDĪŖNa£»A”¢BæÉ·Ö±šÓėC×é³É³£¼ū»ÆŗĻĪļAC”¢AC2”¢BC”¢BC2£¬Ó¦ŹĒC”¢NÓėOŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļ£¬¹ŹAĪŖĢ¼ŌŖĖŲ”¢BĪŖNŌŖĖŲ”¢CĪŖOŌŖĖŲ£»ÓÉĶ¼æÉÖŖFĪŖĘųĢ壬½įŗĻŌ×ÓŠņŹżæÉÖŖ£¬E”¢F“¦ÓŚµŚČżÖÜĘŚ£¬µ„ÖŹ·Šµć×īøߣ¬Ó¦ĪŖŌ×Ó¾§ĢåSi£¬¹ŹEĪŖAl”¢FĪŖCl£¬¾Ż“Ė½ā“š£®
½ā“š ½ā£ŗA”¢B”¢C”¢D”¢E”¢FŹĒŌŖĖŲÖÜĘŚ±ķÖŠĒ°20ŗÅŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®DŌŖĖŲµÄŃęÉ«·“Ó¦³Ź»ĘÉ«£¬ŌņDĪŖNa£»A”¢BæÉ·Ö±šÓėC×é³É³£¼ū»ÆŗĻĪļAC”¢AC2”¢BC”¢BC2£¬Ó¦ŹĒC”¢NÓėOŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļ£¬¹ŹAĪŖĢ¼ŌŖĖŲ”¢BĪŖNŌŖĖŲ”¢CĪŖOŌŖĖŲ£»ÓÉĶ¼æÉÖŖFĪŖĘųĢ壬½įŗĻŌ×ÓŠņŹżæÉÖŖ£¬E”¢F“¦ÓŚµŚČżÖÜĘŚ£¬µ„ÖŹ·Šµć×īøߣ¬Ó¦ĪŖŌ×Ó¾§ĢåSi£¬¹ŹEĪŖAl”¢FĪŖCl£¬
£Ø1£©AĪŖĢ¼ŌŖĖŲ£¬ÓŠ2øöµē×Ó²ć£¬×īĶā²ćµē×ÓŹżĪŖ4£¬“¦ÓŚŌŖĖŲÖÜĘŚ±ķÖŠµŚ¶žÖÜĘŚ¢ōA×壬¹Ź“š°øĪŖ£ŗµŚ¶žÖÜĘŚ¢ōA×壻
£Ø2£©N2·Ö×ÓÖŠNŌ×ÓÖ®¼äŠĪ³É3¶Ō¹²ÓƵē×Ó¶Ō£¬Ęäµē×ÓŹ½ĪŖ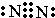 £¬»ÆŗĻĪļCO2µÄ½į¹¹Ź½ĪŖO=C=O£¬µē×Ó²ć½į¹¹ĻąĶ¬ŗĖµēŗÉŹżŌ½“óĄė×Ó°ė¾¶Ō½Š”£¬µē×Ó²ćŌ½¶ąĄė×Ó°ė¾¶Ō½“󣬹ŹĄė×Ó°ė¾¶ÓÉŠ”µ½“óµÄĖ³ŠņĪŖNa+£¼O2-£¼Cl-£¬
£¬»ÆŗĻĪļCO2µÄ½į¹¹Ź½ĪŖO=C=O£¬µē×Ó²ć½į¹¹ĻąĶ¬ŗĖµēŗÉŹżŌ½“óĄė×Ó°ė¾¶Ō½Š”£¬µē×Ó²ćŌ½¶ąĄė×Ó°ė¾¶Ō½“󣬹ŹĄė×Ó°ė¾¶ÓÉŠ”µ½“óµÄĖ³ŠņĪŖNa+£¼O2-£¼Cl-£¬
¹Ź“š°øĪŖ£ŗ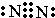 £»O=C=O£»Na+£¼O2-£¼Cl-£»
£»O=C=O£»Na+£¼O2-£¼Cl-£»
£Ø3£©O”¢ClµÄµ„ÖŹ»ņĮ½ŌŖĖŲÖ®¼äŠĪ³ÉµÄ»ÆŗĻĪļæÉ×÷Ė®Ļū¶¾¼ĮµÄÓŠ£ŗO3”¢Cl2”¢ClO2µČ£¬¹Ź“š°øĪŖ£ŗO3”¢Cl2µČ£»
£Ø4£©»ÆŗĻĪļYÓÉO”¢AlĮ½ŌŖĖŲ×é³ÉĪŖAl2O3£¬½«Al2O3”¢N2ÓėC°“1£ŗ1£ŗ3ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦æɵƵ½ZŗĶCO£¬Ó¦ĪŖAl2O3+N2+3CØTZ+3CO£¬ÓÉŌ×ÓŹŲŗćæÉÖŖ£¬ZĪŖAlN£¬
¹Ź“š°øĪŖ£ŗAlN£»
£Ø5£©DµÄ×īøß¼ŪŃõ»Æ¶ŌÓ¦Ė®»ÆĪļĪŖNaOH£¬EµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļĪŖAl£ØOH£©3£¬¶žÕß·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗ£¬
¹Ź“š°øĪŖ£ŗOH-+Al£ØOH£©3ØTAlO2-+H2O£®
µćĘĄ ±¾Ģāæ¼²é½į¹¹ŠŌÖŹĪ»ÖĆ¹ŲĻµÓ¦ÓĆ£¬×¢ŅāµŚČżÖÜĘŚÖŠŌ×Ó¾§ĢåSi·Šµć×īøߣ¬ŹģĮ·ÕĘĪÕŌŖĖŲ»ÆŗĻĪļÖŖŹ¶£¬×¢Ņā»ł“”ÖŖŹ¶µÄČ«ĆęÕĘĪÕ£®


 ½ĢѧĮ·ŠĀĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
½ĢѧĮ·ŠĀĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ČÜŅŗÖŠOH-ÅضČĪŖ0.03mol•L-1 | |
| B£® | øĆČÜŅŗÖŠŗ¬Na+øöŹżĪŖ0.015NA | |
| C£® | ĻņŌČÜŅŗÖŠ¼ÓČė470mLÕōĮóĖ®¼“æÉ | |
| D£® | øĆČÜŅŗÖŠŗ¬ÓŠŃõŌ×ÓøöŹż“óÓŚ0.015NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ±ķĪŖŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬Ēė²ĪÕÕŌŖĖŲ¢Ł-¢ąŌŚ±ķÖŠµÄĪ»ÖĆ£¬ÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧÓĆÓļ»Ų“šĻĀĮŠĪŹĢā£ŗ
±ķĪŖŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬Ēė²ĪÕÕŌŖĖŲ¢Ł-¢ąŌŚ±ķÖŠµÄĪ»ÖĆ£¬ÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧÓĆÓļ»Ų“šĻĀĮŠĪŹĢā£ŗ| ×å ÖÜĘŚ | IA | 0 | ||||||
| 1 | ¢Ł | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | |
| 2 | ¢Ś | ¢Ū | ¢Ü | |||||
| 3 | ¢Ż | ¢Ž | ¢ß | ¢ą | ||||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
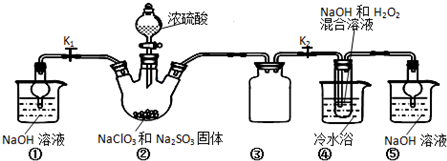
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1molCH3+£Ø¼×»łĢ¼ÕżĄė×Ó£©ŗ¬ÓŠµÄµē×ÓŹżÄæĪŖ9NA | |
| B£® | 27g AlŌŚ±ź×¼×“æöĻĀµÄ22.4L Cl2ÖŠČ¼ÉÕ£¬×ŖŅʵĵē×Ó×ÜŹżĪŖ3 NA | |
| C£® | 0.2 mol•L-1µÄNa2CO3ČÜŅŗÖŠŗ¬ÓŠCO32-µÄŹżÄæŅ»¶ØŠ”ÓŚ0.2NA | |
| D£® | 7.8gNa2SŗĶNa2O2µÄ»ģŗĻĪļÖŠŗ¬ÓŠµÄŅõĄė×Ó×ÜŹżĪŖ0.1 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĖįÓź | B£® | øÉŗµ | C£® | ³ōŃõ²ćæÕ¶“ | D£® | ĪĀŹŅŠ§Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaCl | B£® | NaCl”¢NaBr”¢NaI | C£® | NaBr”¢NaI | D£® | NaI |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
 Ō×ÓŠņŹżŅĄ“ĪŌö“óµÄA”¢B”¢C”¢D”¢E”¢FĮłÖÖŌŖĖŲ£®ĘäÖŠAµÄ»łĢ¬Ō×ÓÓŠ3øö²»Ķ¬Äܼ¶£¬ø÷Äܼ¶ÖŠµÄµē×ÓŹżĻąµČ£»CµÄ»łĢ¬Ō×Ó2pÄܼ¶ÉĻµÄĪ“³É¶Ōµē×ÓŹżÓėAŌ×ÓµÄĻąĶ¬£»DĪŖĖüĖłŌŚÖÜĘŚÖŠŌ×Ó°ė¾¶×ī“óµÄÖ÷×åŌŖĖŲ£»E”¢FŗĶCĪ»ÓŚĶ¬Ņ»Ö÷×壬F“¦ÓŚµŚŅ»øö³¤ÖÜĘŚ£®
Ō×ÓŠņŹżŅĄ“ĪŌö“óµÄA”¢B”¢C”¢D”¢E”¢FĮłÖÖŌŖĖŲ£®ĘäÖŠAµÄ»łĢ¬Ō×ÓÓŠ3øö²»Ķ¬Äܼ¶£¬ø÷Äܼ¶ÖŠµÄµē×ÓŹżĻąµČ£»CµÄ»łĢ¬Ō×Ó2pÄܼ¶ÉĻµÄĪ“³É¶Ōµē×ÓŹżÓėAŌ×ÓµÄĻąĶ¬£»DĪŖĖüĖłŌŚÖÜĘŚÖŠŌ×Ó°ė¾¶×ī“óµÄÖ÷×åŌŖĖŲ£»E”¢FŗĶCĪ»ÓŚĶ¬Ņ»Ö÷×壬F“¦ÓŚµŚŅ»øö³¤ÖÜĘŚ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Na2SO4ČÜŅŗ | B£® | FeCl3ČÜŅŗ | C£® | Cu£ØNO3£©2ČÜŅŗ | D£® | Ļ”ĮņĖį |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com