
���� ��1��������֪�ķ�Ӧ�ó�C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l���ķ�Ӧ�ȣ��������ʵ����뷴Ӧ�ų������������������
��2����ӦΪO3����I-����I2�����ݸ�˹���ɢ�+��+�ۿɵ��ܷ�Ӧ�Լ���H��
��� �⣺��1����H2O��g���TH2O��l����H1=-Q1kJ•mol-1��Q1��0����
��C2H5OH��g���TC2H5OH��l����H2=Q2kJ•mol-1��Q2��0����
��C2H5OH��g��+3O2��g���T2CO2��g��+3H2O��g����H3=-Q3kJ•mol-1��Q3��0����
���ݸ�˹���ɿ�֪���١�3-��+�۵�C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l����H=��-3Q1+Q2-Q3��kJ/mol��
��1molҺ̬�Ҵ���ȫȼ�ղ��ָ������£���ų�������Ϊ��3Q1-Q2+Q3��kJ��
�ʴ�Ϊ����3Q1-Q2+Q3����
��2����������������Ӧ����+��+�ۿɵ��ܷ�Ӧ��2I-��aq��+O3��g��+2H+��aq��=I2��aq��+O2��g��+H2O��l������H=��H1+��H2+��H3��
�ʴ�Ϊ��2I-+O3+2H+=I2+O2+H2O����H1+��H2+��H3��
���� ���⿼��Ӧ�ø�˹���ɼ��㷴Ӧ�ȡ���ѧ����ʽ����д���Ƚϻ�����ע��Ի���֪ʶ���������գ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
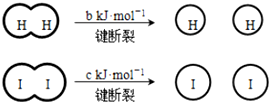 ��a��b��c�������㣩 ����˵������ȷ���ǣ�������
��a��b��c�������㣩 ����˵������ȷ���ǣ�������| A�� | ��Ӧ�������������������������� | |
| B�� | �Ͽ� 1 mol H-H ����1 mol I-I�������������ڶϿ� 2 mol H-I���������� | |
| C�� | �Ͽ� 2 mol H-I����������ԼΪ��c+b+a�� kJ | |
| D�� | ���ܱ������м���2 mol H2��2 mol I2����ַ�Ӧ��ų�������С�� 2a kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���Ӵ�3s�ܼ�ԾǨ��3p�ܼ��γɵĹ����Ƿ������ | |
| B�� | ���ף�P4����������������ṹ���ʷ����еļ���Ϊ109��28�� | |
| C�� | NO2- ����ԭ�Ӳ�ȡsp2�ӻ������ӿռ乹��Ϊ��V���� | |
| D�� | ԭ�Ӿ����۵㲻һ���Ƚ�������ߣ����Ӿ����۵㲻һ���Ƚ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
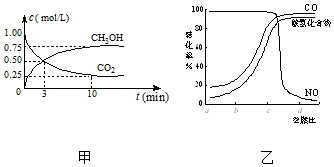 CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | �٢ڢ� | C�� | �٢ڢܢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
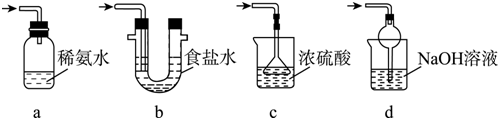
�鿴�𰸺ͽ���>>
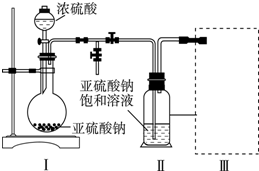
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
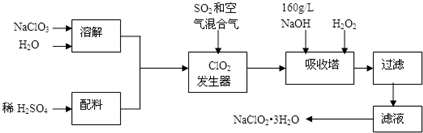
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢ� | B�� | �ڢۢ٢� | C�� | �ڢ٢ۢ� | D�� | �ۢڢܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com