
����Ŀ�������һˮ�ϰ�����ѧ��ѧ�г�����������ʡ�
��1�������£�ij�о���ѧϰС����������·���֤������Ϊ������ʣ�����Ϊ�������е���__(�����)
������һ������0.10mol/LCH3COOH��Һ��Ȼ�����Һ��pH����pH����1����֤������Ϊ�������
���ô�����Һ��������������ʵ�飬��������Һ������������֤������Ϊ�������
�۽�pH=2��CH3COOH��Һ��ˮϡ��100������pH>4����֤������Ϊ�������
��2����25��ʱ��0.10mol/L��CH3COOH�ĵ����Ϊ1%�������Һ��pH=___���ɴ���������c(H+)ԼΪˮ�������c(H+)��___����
���𰸡��� 3 108
��������
��1��Ҫ֤������Ϊ������ʣ�ֻҪ֤�����Ჿ�ֵ��뼴�ɣ�
��2�����ݵ���ȵĸ��pH�ı���ʽ����������Ũ�ȣ�����ˮ�����ӻ�����ˮ�����������Ũ�ȡ�
(1)������һ������0.10mol/LCH3COOH��Һ��Ȼ�����Һ��pH����pH����1��˵����Һ��c(H+)<c(CH3COOH)������Ჿ�ֵ��룬����֤������Ϊ������ʣ�����ȷ��
����Һ������������Ũ�ȳ����ȣ��ô�����Һ��������������ʵ�飬��������Һ����������˵��������Һ������Ũ��С������������Һ��Ũ���Ƿ����δ֪�����Բ���֤������Ϊ������ʣ��ʴ���
�۽�pH=2��CH3COOH��Һ��ˮϡ��100�����������������룬����Һ��pHӦ��С��4���ʴ��ʴ�Ϊ���٣�
(2)��25��ʱ��0.10mol/L��CH3COOH�ĵ����Ϊ1%����Һ��c(H+)=c����=0.10mol/L��1%=0.001mol/L�������Һ��pH=lgc(H+)=lg0.001=3������Һ��ˮ�������c(H+)=![]() =1011mol/L���ɴ���������c(H+)ԼΪˮ�������c(H+)�ı���=
=1011mol/L���ɴ���������c(H+)ԼΪˮ�������c(H+)�ı���=![]() =108���ʴ�Ϊ��3��108��
=108���ʴ�Ϊ��3��108��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ĿҪ���û�ѧ���Իش����⡣
I.ij�л����ʵ��ʽΪC2H6O���������Dzⶨ����Է������������ⶨ�õ���ͼ1��ʾ������ͼ������ú˴Ź����Ǵ������л���õ���ͼ2��ʾ�ĺ˴Ź�������ͼ��

�Իش��������⣺
��1�����л����������Է�������Ϊ_______________��
��2����д�����л�������Ľṹ��ʽ_______________��
II.������X��һ�����ϣ��ɲ�����ϩ��ױ�Ϊ��Ҫԭ�ϣ�����ͼ·�ߺϳɣ�

��1��д������ϩ��ȡA�Ļ�ѧ����ʽ��___________________________________________
��2������ϩΪԭ���Ƶ���Ȳ�ڶ�����Ӧ��������________________________________
��3��д��B��D��Ӧ����F�Ļ�ѧ����ʽ��________________________________________
��4��д��C��D�Ļ�ѧ����ʽ��__________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
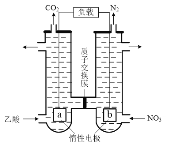
����Ŀ���Ը�����(��Ҫ�ɷ�ΪFeO��Cr2O3��������Al2O3������)Ϊԭ���Ʊ��������Ĺ���������ͼ��ʾ��

��1���������������ù���ΪNa2CrO4��Fe2O3��NaAlO2��
����������ʱFeO��Cr2O3������Ӧ�Ļ�ѧ����ʽΪ____��
����������ʱAl2O3������Ӧ�Ļ�ѧ����ʽΪ____��
��2������pH=7��ʱ����Al(OH)3�����ӷ���ʽΪ____��
��3������������������ΪCr(OH)3������ԭ����Ӧ�����ӷ���ʽΪ____��
��4����ұ����ʱ�Ļ�ѧ����ʽΪ____��
��5������������Է�ˮ����ͼ2��ʾ����������д�������ĵ缫��Ӧʽ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��ϡ��HA��HB��������Һ����ҺpH�ı仯������ͼ��ʾ������V1��ʾϡ��ǰ��������V2��ʾϡ�ͺ���Һ�����������˵����ȷ���ǣ� ��

A.������a��b����![]() ��ֵһ�����
��ֵһ�����
B.pH ��ͬʱ��c(HA)>c(HB)
C.��lg![]() =6ʱ��HA��Һ��pH=8
=6ʱ��HA��Һ��pH=8
D.��֪���������Խǿ�����Ӧ���εļ���Խ������25��ʱ��NaA��Һ��pH һ��С��NaB��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1200 ��ʱ����Ȼ���������лᷢ�����з�Ӧ��H2S(g)��![]() O2(g)= SO2(g)��H2O(g)����H1��H2S(g)��
O2(g)= SO2(g)��H2O(g)����H1��H2S(g)��![]() O2(g)= S(g)��H2O(g)����H2��2H2S(g)��SO2(g)=
O2(g)= S(g)��H2O(g)����H2��2H2S(g)��SO2(g)= ![]() S2(g)��2H2O(g)����H3��2S(g)= S2(g)����H4 ����H4����ȷ����ʽΪ�� ��
S2(g)��2H2O(g)����H3��2S(g)= S2(g)����H4 ����H4����ȷ����ʽΪ�� ��
A.��H4��![]() (��H1����H3��3��H2)B.��H4��
(��H1����H3��3��H2)B.��H4��![]() (3��H2����H1����H3)
(3��H2����H1����H3)
C.��H4��![]() (��H1����H3��3��H2)D.��H4��
(��H1����H3��3��H2)D.��H4��![]() (��H1����H3��3��H2)
(��H1����H3��3��H2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ֱ���ŷź�SO2���������γ����꣬Σ����������ҵ�ϳ����ô���ԭ���ͼ����շ�����SO2���塣
��1����ͼ��ʾ��1molCH4��ȫȼ��������̬ˮ�������仯��1molS(g)ȼ�յ������仯��
�ڴ��������£�CH4���Ի�ԭSO2���ɵ���S(g)��H2O(g)��CO2��д���÷�Ӧ���Ȼ�ѧ����ʽ___��
��2����̿����ԭ��������Ļ�ѧ����ʽΪ2C(s)+2SO2(g)![]() S2(g)+2CO2(g)��һ��ѹǿ�£���1L�ܱ������г��������Ľ�̿��1molSO2������Ӧ�����SO2���������ʺ�S2(g)�������������¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��
S2(g)+2CO2(g)��һ��ѹǿ�£���1L�ܱ������г��������Ľ�̿��1molSO2������Ӧ�����SO2���������ʺ�S2(g)�������������¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��

��A��B��C��D�ĵ��Ӧ��״̬�У��ﵽƽ��״̬����___(����ĸ)��
�ڸ÷�Ӧ����H___0(������������������=��)
�����д�ʩ�ܹ�����SO2ƽ��ת���ʵ���___��
A.�����¶� B.����C���� C.��С������� D.���Ӹ�Ч����
��3���ð�ˮ����SO2��25��ʱ2.0mol��L-1�İ�ˮ�У�NH3��H2O�ĵ��������___(����![]() ��100%)��������SO2������ͨ��ð�ˮ�У�����Һ������ʱ����Һ�е�
��100%)��������SO2������ͨ��ð�ˮ�У�����Һ������ʱ����Һ�е�![]() =___��(��֪25�棬Kb(NH3��H2O)��1.8��10-5��Ka1(H2SO3)��1.3��10-2��Ka2(H2SO3)��6.2��10-8)
=___��(��֪25�棬Kb(NH3��H2O)��1.8��10-5��Ka1(H2SO3)��1.3��10-2��Ka2(H2SO3)��6.2��10-8)
��4��������Һʧȥ����������ͨ��O2�ɵõ�NH4HSO4��Һ������ͼ��ʾװ�õ������NH4HSO4��Һ���Ƶ�ǿ������(NH4)2S2O8��
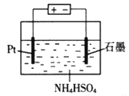
д�����NH4HSO4��Һ�Ļ�ѧ����ʽ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������ѧϰ��ѧ�Ĺ��ߺͻ����������йػ�ѧ�����ʹ����ȷ����
A.ʳ�׳����Ե�ԭ���ǣ�CH3COOH��H2O��CH3COO����H3O��
B.������Һ�ʼ��Ե�ԭ���ǣ�CO32����2H2O![]() H2CO3��2OH��
H2CO3��2OH��
C.��������������ⱥ��ʳ��ˮ�����ӷ���ʽ��Fe��2H2O![]() Fe(OH)2��H2��
Fe(OH)2��H2��
D.��ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ��2H2(g)��O2(g)��2H2O(l)��![]() H����571.6KJ��mol��1
H����571.6KJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��25��ʱ�����з�Ӧ��ƽ�ⳣ�����£�
��N2(g)��O2(g)![]() 2NO(g) K1��1��10-30
2NO(g) K1��1��10-30
��2H2(g)��O2(g)![]() 2H2O(g) K2��2��1081
2H2O(g) K2��2��1081
��2CO2(g)![]() 2CO(g)��O2(g) K3��4��10-92
2CO(g)��O2(g) K3��4��10-92
����˵����ȷ����
A��NO�ֽⷴӦNO(g)![]()
![]() N2(g)��
N2(g)��![]() O2(g)��ƽ�ⳣ��Ϊ1��10-30
O2(g)��ƽ�ⳣ��Ϊ1��10-30
B������K2��ֵ�����жϳ�����H2��O2������Ӧ����H2O
C�������£�NO��H2O��CO2�������ʷֽ�ų�O2������˳��ΪNO>H2O>CO2
D���¶����ߣ�����������Ӧ��ƽ�ⳣ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�������裬��������Ͷ�����̼���������������ѧ���ʾ���һ���������ԣ�þ���ƵĻ�ѧ����Ҳ����һ���������ԣ�
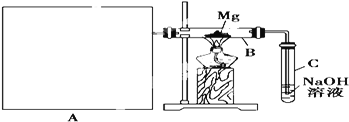
��![]() ������ͼ��ʾװ�ý���þ�Ͷ��������ʵ�飬����A���Ʊ���������ķ���װ�á�
������ͼ��ʾװ�ý���þ�Ͷ��������ʵ�飬����A���Ʊ���������ķ���װ�á�
(1)ѡ����ȡ��������ĺ����Լ�_____________________________![]() �����
�����![]() ��
��
��![]() ��������Һ��
��������Һ��![]() ������Һ���������ƹ����������ƹ���
������Һ���������ƹ����������ƹ���
(2)д��װ��B�з����û���Ӧ�Ļ�ѧ����ʽ��_____________________________װ��C������������Һ��������_____________________________
(3)����ͼ�л����Ʊ���������ķ���װ�ã�ע���������ƣ��̶�����ʡ�Բ�����_______________
(4)����Ϊ��װ�õIJ���֮��____________________________________________________![]() ��д2��
��д2��![]()
��![]() ij�о���ѧϰС���������ʵ�����ƹ������о�����������֪���ٹ�ҵ���ڸ���ʱ��̼��ԭ����������Ƶù裻��þ�ڵ�ȼ�������¼�����������跴Ӧ���۽����軯����ϡ���ᷴӦ���������������⻯�裻�����⻯���ڿ�������ȼ��
ij�о���ѧϰС���������ʵ�����ƹ������о�����������֪���ٹ�ҵ���ڸ���ʱ��̼��ԭ����������Ƶù裻��þ�ڵ�ȼ�������¼�����������跴Ӧ���۽����軯����ϡ���ᷴӦ���������������⻯�裻�����⻯���ڿ�������ȼ��
������ʵ�鱨���м�¼�ţ���![]() ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡ�����ܽ������Ȼ����ˣ�ϴ�ӣ����������
ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡ�����ܽ������Ȼ����ˣ�ϴ�ӣ����������![]() ����ϡ�����ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��
����ϡ�����ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��![]() ���ң���
���ң���
(5)��С����ʵ�����ƹ����Ļ�ѧ����ʽ��_____________________________
(6)���������ϡ�����ܽ�������ʱ�������б������ͻ���ԭ����_____________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com