
���� ��1������n=$\frac{m}{M}$��϶�����̼���ӹ��ɽ��
��2�����ݻ����������������ʵ�����M=$\frac{m}{n}$�����ƽ��Ħ������������ƽ��Ħ���������������һ����̼���ʵ���֮�Ⱥ���ԭ����������
��3������������Ϊǿ����ʣ���ȫ���룬1mol�������������2mol�����Ӻ�3mol��������ӽ��
��� �⣺��1��2.2g CO2���ʵ���Ϊ$\frac{2.2g}{44g/mol}$=0.05mol��������ԭ����ĿΪ0.05mol��2��NA=0.1NA��������Ϊ��0.05mol��22��NA=1.1NA��
�ʴ�Ϊ��0.1NA�� 1.1 NA��
��2�������8.96L ��CH4��CO������壬���ʵ��� Ϊ$\frac{8.96L}{22.4L/mol}$=0.4mol��ƽ��Ħ������Ϊ��$\frac{7.60g}{0.4mol}$=19g/mol��
��������ʵ���Ϊxmol��һ����̼���ʵ���Ϊymol����x+y=0.4��16x+28y=19����ã�x=0.3��y=0.1�����ԣ�CH4��CO���ʵ���֮��Ϊ3��1��Hԭ�ӵ���������0.3mol��4=1.2g��
�ʴ�Ϊ��19g/mol�� 3��1��1.2g��
��3����֪100mLAl2��SO4��3��Һ��Al3+Ũ��Ϊ0.6mol/L������SO42-��Ũ��Ϊ��0.6mol/L��$\frac{3}{2}$=0.9mol/L��
�����������ʵ���Ϊ��0.1L��0.6mol/L��$\frac{1}{2}$=0.03mol��
�ʴ�Ϊ��0.9 mol/L�� 0.03mol��
���� ���⿼�����ʵ����ļ��㣬Ϊ��Ƶ���㣬�������ʵ���Ϊ���ĵĻ������㹫ʽΪ���Ĺؼ������ط�������������Ŀ��飬��Ŀ�ѶȲ���


 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | I-��Fe2+��Cl-��SO2 | B�� | Cl-��Fe2+��SO2��I- | C�� | Fe2+��I-��Cl-��SO2 | D�� | SO2��I-��Fe2+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ϡ���� | C�� | NaOH��Һ | D�� | FeCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O | B�� | ŨH2SO4 | C�� | NaCl | D�� | NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
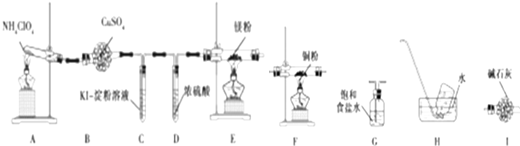
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

| ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
| A | ϡ���� | Cu | NO | H2O |
| B | ϡH2SO4 | CaCO3 | CO2 | NaOH��Һ |
| C | Ũ��ˮ | NaOH���� | NH3 | H2O |

| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| ������g�� | m1=5.4 | m2=8.2 | m3=7.4 | m4=7.2 | m5=7.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
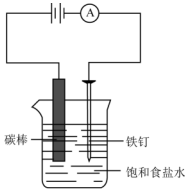 ��ͼ��ʾ���ش��������⣺
��ͼ��ʾ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.964g/L | B�� | 0.5091g/L | C�� | 1.964g/mL | D�� | 0.5091g/mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ag+��K+��NO3-��Cl - | B�� | K+��Cu2+��SO42-��HCO3- | ||
| C�� | Mg2+��Cl-��SO42-��Na+ | D�� | CO32-��K+��NO3-��Na+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com