
����Ŀ��һ��������������(CH3CH2CH2CH2CH3)���������ѽⷴӦ��
��.CH3CH2CH2 CH2CH3 (g)CH3CH===CH2(g)��CH3 CH3 (g) ��H1����274.2 kJ��mol��1
��.CH3CH2CH2CH2CH3(g)CH3CH2CH3(g)��CH2===CH2(g) ��H2����122.7 kJ��mol -1
�ش��������⣺
��1���ں��º�ѹ���ܱ������У�����һ�����������鷢���ѽⷴӦ����ʼʱ�������Ϊ a L��һ��ʱ�䷴Ӧ�ﵽƽ������������Ϊ b L����ʱ�������ת���� ��(������)��_________����Ӧ��ϵ�г���һ������ˮ����(ˮ �����ڸ������²����뷴Ӧ)���ٴ�ƽ����������ת���ʽ�_____(��������������С������������)��ԭ��Ϊ_______________��
��2���¶�Ϊ T ��ʱ����ѹǿ��Ϊ 100 kPa ���ܱ������г��� 1 mol��L��1 CH3CH=CH2�� 2 mol��L��1 CH3CH3������Ӧ��CH3CH===CH2(g)��CH3CH3(g) CH3CH2CH3(g)��CH2===CH2(g)��H3����� CH3CH2CH3 �����ʵ���Ũ����ʱ�� t �ı仯��ͼ����������ʾ��
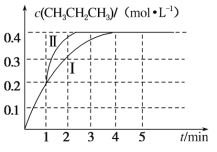
����H3��_____��
�ڸ÷�Ӧ��ƽ�ⳣ�� Kp��_____��(Kp Ϊ�Է�ѹ��ʾ��ƽ�ⳣ������ѹ����ѹ�����ʵ��������������� ���� 2 λС��)��
������ 1 min ʱ���ı�ijһ��Ӧ��������������Ϊ����������ı������Ϊ_____��
��3���� 0.1 mol CH3CH3��ȫȼ�պ������ͨ�� 100 mL 3 mol��L��1�� NaOH ��Һ�У���ַ�Ӧ��������Һ������Ũ�ȵĴ�С˳��Ϊ_____��
��4����ϡ����Ϊ�������Һ��CH3CH3 ȼ�ϵ�صĸ�����ӦʽΪ_____��
���𰸡�![]() ���� ����ˮ������������������൱�ڼ�ѹ������ƽ�������ƶ� -151.5 kJ��mol��1 0.17 �����Ч���� c��Na+����c��HCO3-����c��CO32-����c��OH-����c(H+)
���� ����ˮ������������������൱�ڼ�ѹ������ƽ�������ƶ� -151.5 kJ��mol��1 0.17 �����Ч���� c��Na+����c��HCO3-����c��CO32-����c��OH-����c(H+) ![]()
��������
��1�����������鷢���������ѽⷴӦ�ķ���ʽ��1L�������ѽ�����2L���壬��1L��������ȫ�ѽ⣬�������1L����Ӧ��ϵ�г���һ������ˮ��������������൱�ڼ�ѹ��
��2���ٸ��ݸ�˹���ɼ���CH3CH==CH2(g)��CH3CH3(g)![]() CH3CH2CH3(g)��CH2==CH2(g)���ʱ䣻
CH3CH2CH3(g)��CH2==CH2(g)���ʱ䣻
������������ʽ������ƽ�ⳣ����
��1 min ʱ���ı�ijһ��Ӧ��������Ӧ���ʼӿ죬ƽ��û���ƶ���
��3��0.1 mol CH3CH3��ȫȼ������0.2mol������̼���壬ͨ�� 100 mL 3 mol��L��1�� NaOH ��Һ�У�����̼Ԫ�ء���Ԫ���غ㣬��֪����0.1mol Na2CO3��0.1 mol NaHCO3��
��4����ϡ����Ϊ�������Һ�� CH3CH3�ڸ���ʧ�������ɶ�����̼���塣
��1�����������鷢���������ѽⷴӦ�ķ���ʽ��1 L�������ѽ�������2 L���壬��1L�������ѽ⣬�������1 L�����Ϊ a L��������ﵽƽ������������Ϊ b L���������(b-a)L����ֽ������������Ϊ(b-a)L���������ת���� ��(������)��![]() ����Ӧ��ϵ�г���һ������ˮ��������������൱�ڼ�ѹ��ƽ�������ƶ����������ת��������
����Ӧ��ϵ�г���һ������ˮ��������������൱�ڼ�ѹ��ƽ�������ƶ����������ת��������
��2������.CH3CH2CH2 CH2CH3 (g)![]() CH3CH=CH2(g)��CH3 CH3 (g) ��H1����274.2 kJ��mol��1��
CH3CH=CH2(g)��CH3 CH3 (g) ��H1����274.2 kJ��mol��1��
��.CH3CH2CH2CH2CH3(g)![]() CH3CH2CH3(g)��CH2=CH2(g) ��H2����122.7 kJ��mol -1��
CH3CH2CH3(g)��CH2=CH2(g) ��H2����122.7 kJ��mol -1��
���ݸ�˹������������CH3CH=CH2(g)��CH3CH3(g)![]() CH3CH2CH3(g)��CH2=CH2(g) ��H3=��122.7 kJ��mol -1��274.2 kJ��mol��1= ��151.5 kJ��mol��1��
CH3CH2CH3(g)��CH2=CH2(g) ��H3=��122.7 kJ��mol -1��274.2 kJ��mol��1= ��151.5 kJ��mol��1��
��
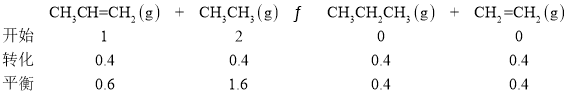
Kp��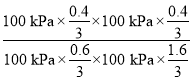 ==0.17
==0.17
��1 min ʱ���ı�ijһ��Ӧ��������Ӧ���ʼӿ죬ƽ��û���ƶ������Ըı�������Ǽ����Ч������
��3��0.1 mol CH3CH3��ȫȼ������0.2 mol CO2���壬ͨ�� 100 mL 3 mol��L��1�� NaOH ��Һ�У�����̼Ԫ�ء���Ԫ���غ㣬��֪����0.1 mol Na2CO3��0.1mol NaHCO3��̼����ˮ��̶ȴ���̼�����ƣ�������Һ������Ũ�ȵĴ�С˳��Ϊc��Na+����c��HCO3-����c��CO32-����c��OH-����c(H+)��
��4����ϡ����Ϊ�������Һ�� CH3CH3�ڸ���ʧ�������ɶ�����̼���壬������Ӧʽ��![]() ��
��


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���X�Ľṹ��ͼ��ʾ������˵����ȷ����

A.��ʹ���Ը��������Һ��ɫ
B.X�����к���2������̼ԭ��
C.����FeCl3��Һ������ɫ��Ӧ
D.1 mol X�������5 mol H2�����ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС����˽̰�̲�ʵ�顰��200mL�ձ��з���20g���ǣ�C12H22O11������������ˮ��������ȣ�Ȼ���ټ���15mL��������Ϊ98%Ũ���ᣬѸ�ٽ��衱��������̽����
��1���۲����������ȱ�ƣ�����ڣ�������ͣ��γ����ɶ�ĺ���״��ɫ���ʣ�ͬʱ�ŵ��̼�����ζ����ѹ�˺�ɫ����ʱ���о���Ӳ������ˮ�г�Ư��״̬��ͬѧ�������������Ʋ�����н��ۣ�
��Ũ�������ǿ������ ��Ũ���������ˮ�� ��Ũ���������ˮ�Ԣ�Ũ����������� �ݺ�ɫ���ʾ���ǿ������
�������ݲ���ֵ���_________������ţ���
��2��Ϊ����֤������Ũ���ᷴӦ���ɵ���̬���ͬѧ�����������װ�ã�

�Իش��������⣺
��ͼ1��A�����ѡ������װ��_________�����ţ���
��ͼ1�� Bװ����װ�Լ���_________��Dװ�����Լ���������_________��Eװ���з�����������_________��
��ͼ1��Aװ����ʹ�����ȱ�ڵĻ�ѧ��Ӧ����ʽΪ_________����������͵Ļ�ѧ����ʽΪ��_________��
��ijѧ����ͼ2����ʵ��ʱ������DƿƷ�첻��ɫ��Eװ�����������ݳ���Fװ�������Ը��������Һ��ɫ��dz���Ʋ�Fװ�������Ը��������Һ��ɫ��dz��ԭ��_________���䷴Ӧ�����ӷ���ʽ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ұ���ķ���������Ϊԭ����ȡ��ϸ��-���������Ƚ��ͻ�����Ⱦ�ֿ��������Դ�������ʡ���֪���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3�������Ʊ�ʵ���������£�
��1�������������������ᷴӦ�Ļ�ѧ����ʽΪ ��
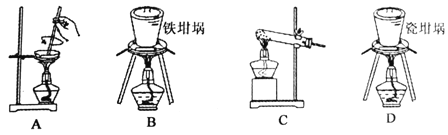
��2����ͼ������������NaOH�����Ʊ������ƣ��ɲ��õ�װ��Ϊ ����ѡ���ţ���
��3����ʵ�������У���30%��H2O2��Һ���������ӷ�Ӧ����ʽΪ ��
��4����֤������������Һ���Ƿ��������ӵIJ�������Ϊ ��
��5�����õ���pH��Һ������������õ�Fe(OH)3����֪��25��ʱ��Ksp[Fe(OH)3]=4.0��10-38������¶��·�ӦFe3++3H2O![]() Fe(OH)3+3H+��ƽ�ⳣ��Ϊ ��
Fe(OH)3+3H+��ƽ�ⳣ��Ϊ ��
��6������������茶��壬��������Ҫ��ӦΪ��4[NH4Al(SO4)2��12H2O]![]() 2Al2O3+ 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ����ͼ��ʾ��װ�á�
2Al2O3+ 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ����ͼ��ʾ��װ�á�

������ƿ���ռ����������� (�ѧʽ)��
��KMnO4��Һ��ɫ�����������ӷ�Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ijѧ����0.1 mol��L��1H2SO4��Һ�ζ�0.1 mol��L��1NaOH��Һ���кͺ��ˮ��100 mL�����ζ��յ���ж��������ٵ���һ��H2SO4��Һ���ڶ����һ��H2SO4��Һ(1��Ϊ0.05 mL)����ٺ͢����������������Һ��pH֮����(����)
A. 4B. 4.6C. 5.4D. 6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����������![]() ��Ҫ�ɷ�Ϊ
��Ҫ�ɷ�Ϊ![]() ������������
������������![]() ������
������![]() ��ȡ��������ұ������ԭ�ϣ���ȡ�IJ����������£�
��ȡ��������ұ������ԭ�ϣ���ȡ�IJ����������£�
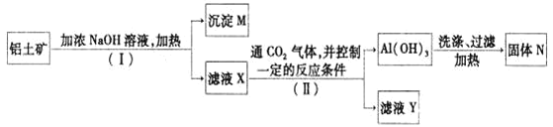
(1)![]() ��II�����з�����Һ�ͳ����IJ���Ϊ_________�����õ��IJ��������ǣ�________��
��II�����з�����Һ�ͳ����IJ���Ϊ_________�����õ��IJ��������ǣ�________��
(2)����M�г�������ɳ�⣬һ��������_______������N��_______��
(3)��ҺX�У�����Ԫ�ص����ʵĻ�ѧʽΪ______��������_____![]() ������������������������
������������������������![]() �����ʣ�
�����ʣ�
(4)ʵ�����ﳣ��![]() ��Һ�м���___________
��Һ�м���___________![]() ������ˮ������NaOH��Һ��
������ˮ������NaOH��Һ��![]() ����ȡ
����ȡ![]() ��
��
(5)�����ۺ��������Ļ�����ȼ����Ӧ�ų����������������ɵ�Һ̬���������������졣��д����Ӧ�Ļ�ѧ����ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(MoS2�� ����Mo�Ļ��ϼ�Ϊ+4)����Ϊ��������֮���������õ�Ʒ�ʵĻ����(��MoS2��SiO2 �Լ�CuFeS2������)�Ʊ��ߴ��������һ�������������£�

�ش��������⣺
(1)��������м����������Ϊ�˳�ȥ����SiO2���÷�Ӧ�Ļ�ѧ����ʽΪ___________��
(2)����������������������Ҫ�ǽ�MoS2ת��ΪMoO3���ڸ÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��
(3)���������ղ�������ս���������������ǰ������з��鴦������Ŀ����_________����������������( NH4)2 MoO4��Ӧ�Ļ�ѧ����ʽΪ___________��
(4)���������������Һ�м���Na2S�������ת��Ϊ��������[(NH4)2MoS4]�����������(NH4)2 MoS4�����ᷴӦ����MoS3������������Ӧ�����ӷ���ʽΪ_________________��
(5)�ߴ�MoS2����Ȼ����ڼ����ķ����Ⱦ���MoS2.8�����ʣ��ڸ�������Ϊ���ֵ����ԣ�MoԪ����+4��+6���ּ�̬����MoS2��Mo4+��ռMoԪ�ص����ʵ�������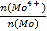 Ϊ__________��
Ϊ__________��
(6)�����ƾ���( Na2 MoO4 2H2O)��һ����������ȴˮϵͳ������ʴ����������MoS2�Ʊ������Ʊ������������Ba(OH)2�����ȥSO42-������Һ��c(MoO42-)=0.4 mol/L��c(SO42-)=0. 05 mol/L�������£���BaMoO4������ʼ����ʱ��SO42-��ȥ����Ϊ____________ [������Һ����仯����֪��259�棬Ksp( BaMoO4)=4.0��10-8 �� Ksp(BaSO4)=1.1��10-10]��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ɫ��ѧ���������������ཡ��ϢϢ��ص��������⣬�������֪��Ϣ�ش��������⣺
(1)�����Ʊ�������ķ�Ӧ��ԭ�Ӿ�������ߵ���_____(����ĸ)��
A.CH2=CH2+HCl��CH3CH2Cl
B.CH3CH2OH+HCl![]() CH3CH2Cl+H2O
CH3CH2Cl+H2O
C.CH3CH3+Cl2![]() CH3CH2Cl+HCl
CH3CH2Cl+HCl
D.CH2=CHCl+H2![]() CH3CH2Cl
CH3CH2Cl
�������ĸ���Ӧ�ɹ��ɳ���ԭ�Ӿ�������ߵ���______(�Ӧ����)��
(2)�ж����ʵ���������Ҳ����ɫ��ѧ�о�������֮һ��ClO2��һ�����������������������ɽ���ˮ��������CN-���ж������������������ȥ����д����ClO2����ˮ�е�CN-����������������ӷ���ʽ��______���÷������ŵ���_______��
(3)ij����ˮ������Ȼˮ�Ʊ�����ˮ(ȥ����ˮ)�Ĺ�������ʾ��ͼ��ͼ��

����̿��������_______��O3�������ŵ���_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij![]() ��Ʒ�к���
��Ʒ�к���![]() ��
��![]() ���ʣ�������ȡ������
���ʣ�������ȡ������![]() ��ijͬѧ�����ͼ��ʵ�鷽������ش��������⣺
��ijͬѧ�����ͼ��ʵ�鷽������ش��������⣺
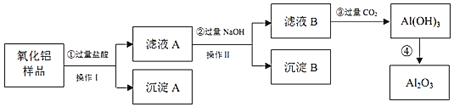
(1)�������������______���ڸò������õ��IJ��������������ձ���������������______
(2)����A�ijɷ���______![]() �ѧʽ
�ѧʽ![]() ��д���ڢ۲���Ӧ����Ԫ��ת�������ӷ���ʽ______
��д���ڢ۲���Ӧ����Ԫ��ת�������ӷ���ʽ______
(3)д��֤����ҺB��![]() �ѳ�����ȫ��ʵ�鷽��______
�ѳ�����ȫ��ʵ�鷽��______
(4)���ı���������ͼ�Ľṹ�������ٹ������������ڹ���NaOH������λ�ã������۹���![]() ��Ӧ��Ϊ_____��д���˷��������ɳ���B�����ӷ���ʽ______
��Ӧ��Ϊ_____��д���˷��������ɳ���B�����ӷ���ʽ______
(5)Ϊ�˵õ����Ӵ�����![]() �����˺���Ҫ���в���������______
�����˺���Ҫ���в���������______
(6)д����ҵ��������ұ�����Ļ�ѧ����ʽ______
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com