
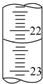
 ŹµŃéŹŅÖŠÓŠŅ»Ī“ÖŖÅØ¶ČµÄĻ”ŃĪĖį£¬Ä³Ń§ÉśÓĆ0.10mol?L-1 NaOH±ź×¼ČÜŅŗ½ųŠŠ²ā¶ØŃĪĖįµÄÅØ¶ČµÄŹµŃé£®Č”20.00mL“ż²āŃĪĖį·ÅČė׶ŠĪĘæÖŠ£¬²¢µĪ¼Ó2”«3µĪ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ÓĆ×Ō¼ŗÅäÖʵÄNaOH±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø£®ÖŲø“ÉĻŹöµĪ¶Ø²Ł×÷2”«3“Ī£¬¼ĒĀ¼Źż¾ŻČēĻĀ£®ĒėĶź³ÉĻĀĮŠĢīæÕ£ŗ
ŹµŃéŹŅÖŠÓŠŅ»Ī“ÖŖÅØ¶ČµÄĻ”ŃĪĖį£¬Ä³Ń§ÉśÓĆ0.10mol?L-1 NaOH±ź×¼ČÜŅŗ½ųŠŠ²ā¶ØŃĪĖįµÄÅØ¶ČµÄŹµŃé£®Č”20.00mL“ż²āŃĪĖį·ÅČė׶ŠĪĘæÖŠ£¬²¢µĪ¼Ó2”«3µĪ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ÓĆ×Ō¼ŗÅäÖʵÄNaOH±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø£®ÖŲø“ÉĻŹöµĪ¶Ø²Ł×÷2”«3“Ī£¬¼ĒĀ¼Źż¾ŻČēĻĀ£®ĒėĶź³ÉĻĀĮŠĢīæÕ£ŗ| ŹµŃ鱹ŗÅ | “ż²āŃĪĖįµÄĢå»ż£ØmL£© | NaOHČÜŅŗµÄÅØ¶Č£Ømol?L-1£© | µĪ¶ØĶź³ÉŹ±£¬NaOHČÜŅŗµĪČėµÄĢå»ż£ØmL£© |
| 1 | 20.00 | 0.10 | 24.18 |
| 2 | 20.00 | 0.10 | 23.06 |
| 3 | 20.00 | 0.10 | 22.96 |
| c(±ź×¼)”ĮV(±ź×¼) |
| V(“ż²ā) |
| 23.06+22.96 |
| 2 |
| c(±ź×¼)”ĮV(±ź×¼) |
| V(“ż²ā) |
| 0.10mol/L”Į23.01mL |
| 20.00mL |


 ĆĻ½ØĘ½“ķĢā±¾ĻµĮŠ“š°ø
ĆĻ½ØĘ½“ķĢā±¾ĻµĮŠ“š°ø ³¬ÄÜѧµäÓ¦ÓĆĢāĢāæØĻµĮŠ“š°ø
³¬ÄÜѧµäÓ¦ÓĆĢāĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĀČŹĒŅ»ÖÖ·Ē³£ÖŲŅŖµÄ·Ē½šŹōŌŖĖŲ£®
ĀČŹĒŅ»ÖÖ·Ē³£ÖŲŅŖµÄ·Ē½šŹōŌŖĖŲ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢±ź×¼×“æöĻĀ£¬4.48LĖ®ÖŠŗ¬ÓŠµÄ·Ö×ÓŹżÄæĪŖ0.2NA |
| B”¢0.1molÄĘÓė×ćĮæĖ®·“Ó¦×ŖŅʵĵē×ÓŹżÄæĪŖ0.1NA |
| C”¢0.2mol?L-1CuSO4ČÜŅŗÖŠŗ¬ÓŠµÄSO42-Ąė×ÓŹżÄæĪŖ0.2NA |
| D”¢0.5molKClO3ÖŠŗ¬ÓŠCl-µÄŹżÄæĪŖ0.5NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Ąė×Ó»ÆŗĻĪļÖŠÖ»“ęŌŚŅõ”¢ŃōĄė×ÓÖ®¼äµÄ¾²µē×÷ÓĆ |
| B”¢ĻąĶ¬ÖŹĮæµÄN2”¢N4”¢N30ŗ¬ÓŠµÄ·Ö×ÓŹżĻąĶ¬ |
| C”¢23gÄĘŗĶ×ćĮæĖ®·“Ó¦Ź§Č„6.02”Į1023øöµē×Ó |
| D”¢µ±1molCl2µÄĢå»żĪŖ22.4LŹ±£¬øĆĀČĘųŅ»¶Ø“¦ÓŚ±ź×¼×“æöĻĀ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
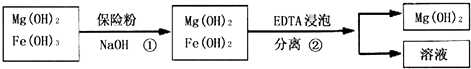
| ¾«ÖĘ×čČ¼¼ĮµÄĢõ¼ž | ×čČ¼¼ĮĢśŗ¬Įæ | |||
| ŠņŗÅ | Ģį“æĢåĻµĪĀ¶Č/”ę | ¼ÓČėEDTAÖŹĮæ/g | ¼ÓČė±£ĻÕ·ŪÖŹĮæ/g | W£ØFe£©/£Ø10-4g£© |
| 1 | 40 | 0.05 | 0.05 | 7.63 |
| 2 | 40 | 0.05 | 0.10 | 6.83 |
| 3 | 60 | 0.05 | 0.10 | 6.83 |
| 4 | 60 | 0.10 | 0.10 | 6.51 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Ō×Ó°ė¾¶¼õŠ” |
| B”¢µēĄėÄÜŌö“ó |
| C”¢µēøŗŠŌŌö“ó |
| D”¢¾łŹĒÖÜĘŚŠŌŌö“ó |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com