
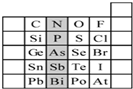
 ŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ£¬·“Ó³ĮĖŌŖĖŲµÄŌ×Ó½į¹¹ŗĶŌŖĖŲµÄŠŌÖŹ£®ĻĀĶ¼ŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£®ĒėÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½ĢīæÕ£ŗ
ŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ£¬·“Ó³ĮĖŌŖĖŲµÄŌ×Ó½į¹¹ŗĶŌŖĖŲµÄŠŌÖŹ£®ĻĀĶ¼ŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£®ĒėÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½ĢīæÕ£ŗ·ÖĪö £Ø1£©¢ŁĶ¬ÖÜĘŚ×Ō×ó¶ųÓŅŌŖĖŲµÄ·Ē½šŹōŠŌŌöĒ棬Ķ¬Ö÷×å×ŌÉĻ¶ųĻĀŌŖĖŲµÄ·Ē½šŹōŠŌ¼õČõ£»
¢ŚĶ¬ÖÜĘŚ×Ō×ó¶ųÓŅŌŖĖŲµÄ½šŹōŠŌ¼õČõ£¬Ķ¬Ö÷×å×ŌÉĻ¶ųĻĀŌŖĖŲµÄ½šŹōŠŌŌöĒ棻
¢ŪĶ¬ÖÜĘŚ×Ō×ó¶ųÓŅŌ×Ó°ė¾¶¼õŠ”£¬Ķ¬Ö÷×å×ŌÉĻ¶ųĻĀŌ×Ó°ė¾¶Ōö“ó£»
¢Ü×īøß¼Ūŗ¬ŃõĖįĖįŠŌ×īĒæµÄŹĒøßĀČĖį£»
¢ŻŌŚ½šŹōŗĶ·Ē½šŹō·Ö½ēĻß“¦µÄŌŖĖŲµ„ÖŹÄÜ×÷°ėµ¼Ģ壻
¢ŽÖ÷×åŌŖĖŲÖŠ£¬Ō×Ó×īĶā²ćµē×ÓŹżÓėĘä×åŠņŹż”¢×īøß»ÆŗĻ¼ŪŹżĻąµČ£»
£Ø2£©¢ŁŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬Ęä×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĖįŠŌŌ½Ē棻
¢ŚŌŖĖŲµÄ½šŹōŠŌŌ½Ē棬ĘäĒā»ÆĪļŌ½ĪČ¶Ø£»
¢Ūŗ¬ÓŠĒā¼üµÄĪļÖŹ·Šµć½Ļøߣ»
¢Ü·Ē½šŹōŠŌŌ½Ē棬ŅõĄė×Ó»¹ŌŠŌŌ½Čõ£»
¢ŻµēøŗŠŌĻą½üµÄŌŖĖŲ£¬Ęä·Ē½šŹōŠŌĻą½ü£»
£Ø3£©¢Łøł¾ŻĶ¬Ņ»Ö÷×åŌŖĖŲŠŌÖŹµÄĻąĖĘŠŌŗĶµŻ±äŠŌ½ā“š£»
¢ŚäåŗĶ¶žŃõ»ÆĮņ·“Ó¦Ńõ»Æ»¹Ō·“Ӧɜ³ÉĮņĖįŗĶĒāäåĖį£®
½ā“š ½ā£ŗ£Ø1£©¢ŁĶ¬ÖÜĘŚ×Ō×ó¶ųÓŅŌŖĖŲµÄ·Ē½šŹōŠŌŌöĒ棬Ķ¬Ö÷×å×ŌÉĻ¶ųĻĀŌŖĖŲµÄ·Ē½šŹōŠŌ¼õČõ£¬·Ē½šŹōŠŌ×īĒæµÄŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÓŅÉĻ½Ē£¬ĪŖFŌŖĖŲ£¬¹Ź“š°øĪŖ£ŗF£»
¢ŚĶ¬ÖÜĘŚ×Ō×ó¶ųÓŅŌŖĖŲµÄ½šŹōŠŌ¼õČõ£¬Ķ¬Ö÷×å×ŌÉĻ¶ųĻĀŌŖĖŲµÄ½šŹōŠŌŌöĒ棬½šŹōŠŌ×īĒæµÄŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķ×óĻĀ½Ē£¬ĪŖPbŌŖĖŲ£¬¹Ź“š°øĪŖ£ŗPb£»
¢ŪĶ¬ÖÜĘŚ×Ō×ó¶ųÓŅŌ×Ó°ė¾¶¼õŠ”£¬Ķ¬Ö÷×å×ŌÉĻ¶ųĻĀŌ×Ó°ė¾¶Ōö“ó£¬Ō×Ó°ė¾¶×īŠ”µÄŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÓŅÉĻ½Ē£¬ĪŖFŌŖĖŲ£¬¹Ź“š°øĪŖ£ŗF£»
¢ÜŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬Ęä×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĖįŠŌŌ½Ē棬FŗĶOŌŖĖŲƻӊ×īøß¼Ūŗ¬ŃõĖį£¬ĖłŅŌ×īøß¼Ūŗ¬ŃõĖįĖįŠŌ×īĒæµÄŹĒHClO4£¬¹Ź“š°øĪŖ£ŗHClO4£»
¢ŻŌŚ½šŹōŗĶ·Ē½šŹō·Ö½ēĻß“¦µÄŌŖĖŲµ„ÖŹÄÜ×÷°ėµ¼Ģ壬ČēSi£¬¹Ź“š°øĪŖ£ŗSi£»
¢ŽÖ÷×åŌŖĖŲÖŠ£¬Ō×Ó×īĶā²ćµē×ÓŹżÓėĘä×åŠņŹż”¢×īøß»ÆŗĻ¼ŪŹżĻąµČ£¬Õā¼øÖÖŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ5£¬ĖłŅŌÕā¼øÖÖŌŖĖŲĪ»ÓŚµŚVA×壬ŠĪ³ÉµÄ×īøß»ÆŗĻ¼ŪĪŖ+5£¬¹Ź“š°øĪŖ£ŗVA£»+5£»
£Ø2£©¢ŁŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬Ęä×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĖįŠŌŌ½Ē棬PŌŖĖŲµÄ·Ē½šŹōŠŌ“óÓŚAsŌŖĖŲ£¬ĖłŅŌĖįŠŌĒæČõ£ŗH3AsO4£¼H3PO4£¬¹Ź“š°øĪŖ£ŗ£¼£»
¢ŚŌŖĖŲµÄ½šŹōŠŌŌ½Ē棬ĘäĒā»ÆĪļŌ½ĪČ¶Ø£¬SµÄ·Ē½šŹōŠŌŠ”ÓŚClŌŖĖŲ£¬ĖłŅŌĪČ¶ØŠŌ£ŗH2S£¼HCl£¬¹Ź“š°øĪŖ£ŗ£¼£»
¢ŪĶ¬Ņ»Ö÷×åÖŠ£¬ŗ¬ÓŠĒā¼üµÄĪļÖŹ·Šµć“óÓŚĘäĻąĮŚŌŖĖŲĒā»ÆĪļµÄ·Šµć£¬HFÖŠŗ¬ÓŠĒā¼ü£¬HCl֊ƻӊĒā¼ü£¬ĖłŅŌ·ŠµćHF£¾HCl£¬¹Ź“š°øĪŖ£ŗ£¾£»
¢ÜĶ¬Ņ»Ö÷×åÖŠ£¬ŅõĄė×ӵĻ¹ŌŠŌĖę×ÅŌ×ÓŠņŹżµÄŌö“ó¶ųŌöĒ棬ĖłŅŌ»¹ŌŠŌI-£¾Br-£¬¹Ź“š°øĪŖ£ŗ£¾£»
¢ŻµēøŗŠŌĻą½üµÄŌŖĖŲ£¬Ęä·Ē½šŹōŠŌĻą½ü£¬OŌŖĖŲŗĶClŌŖĖŲµÄµēøŗŠŌ½Ó½ü£¬ĖłŅŌĘä·Ē½šŹōŠŌ½Ó½ü£¬¹ŹŃ”£ŗD£»
£Ø3£©¢ŁA£®ĮņĪŖ¹ĢĢ壬ĖłŅŌ³£ĪĀĻĀSeµ„ÖŹŹĒ¹ĢĢ壬¹ŹA“ķĪó£»
B£®Se×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĪŖĖį£¬ÄÜŗĶNaOH·¢ÉśÖŠŗĶ·“Ó¦£¬¹ŹBÕżČ·£»
C£®Ķ¬Ņ»Ö÷×åÖŠ£¬ĘäĒā»ÆĪļµÄ»ÆѧŹ½ĻąĶ¬£¬ĖłŅŌSeĒā»ÆĪļµÄ»ÆѧŹ½ĪŖH2Se£¬¹ŹCÕżČ·£»
D£®øł¾ŻĮņµÄŃõ»ÆĪļÖŖ£¬³£¼ūµÄŃõ»ÆĪļÓŠSeO3ŗĶSeO2£¬¹ŹD“ķĪó£®
¹ŹŃ”£ŗBC£»
¢Śäå¾ßÓŠĒæŃõ»ÆŠŌ£¬Äܽ«¶žŃõ»ÆĮņŃõ»ÆĪŖĮņĖį£¬Ķ¬Ź±×ŌÉķ±»»¹ŌĪŖĒāäåĖį£¬Ąė×Ó·“Ó¦·½³ĢŹ½ĪŖBr2+2H2O+SO2ØT4H++SO42-+Cl-£¬
¹Ź“š°øĪŖ£ŗBr2+2H2O+SO2ØT4H++SO42-+Cl-£®
µćĘĄ ±¾Ģāæ¼²éĮĖŌŖĖŲÖÜĘŚ±ķŗĶŌŖĖŲÖÜĘŚĀɵÄ×ŪŗĻÓ¦ÓĆ£¬ŹģĮ·ÕĘĪÕŌŖĖŲÖÜĘŚĀÉŹĒ½ā±¾Ģā¹Ų¼ü£¬×¢Ņā¶Ō»ł“”ÖŖŹ¶µÄĄķ½āÕĘĪÕ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 µŚĖÄÖÜĘŚµÄŠķ¶ą½šŹōÄÜŠĪ³ÉÅäŗĻĪļ£®æĘѧ¼ŅĶعżXÉäĻß²āµĆµØ·Æ½į¹¹Ź¾ŅāĶ¼æɼņµ„±ķŹ¾ČēĻĀ£ŗ
µŚĖÄÖÜĘŚµÄŠķ¶ą½šŹōÄÜŠĪ³ÉÅäŗĻĪļ£®æĘѧ¼ŅĶعżXÉäĻß²āµĆµØ·Æ½į¹¹Ź¾ŅāĶ¼æɼņµ„±ķŹ¾ČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŖĖŲX µÄ¼ņµ„ĘųĢ¬Ēā»ÆĪļµÄČČĪČ¶ØŠŌ±ČW µÄĒæ | |
| B£® | ŌŖĖŲW µÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌ±ČZ µÄČõ | |
| C£® | »ÆŗĻĪļYX”¢ZX2”¢WX3 ÖŠ»Æѧ¼üµÄĄąŠĶĻąĶ¬ | |
| D£® | ŌŖĖŲX µÄ¼ņµ„ĘųĢ¬Ēā»ÆĪļµÄ·Šµć±ČWµÄµĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
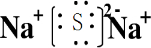 £¬GSO3£»
£¬GSO3£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 4mL | B£® | 10.8mL | C£® | 1.2mL»ņ4mL | D£® | 8mL»ņ10.8mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚ¹²¼Ū»ÆŗĻĪļÖŠŅ»¶Øŗ¬ÓŠ¹²¼Ū¼ü | |
| B£® | ÓÉ·Ē½šŹōŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļŅ»¶ØŹĒ¹²¼Ū»ÆŗĻĪļ | |
| C£® | ŗ¬ÓŠĄė×Ó¼üµÄ»ÆŗĻĪļŅ»¶ØŹĒĄė×Ó»ÆŗĻĪļ | |
| D£® | Ė«Ō×Óµ„ÖŹ·Ö×ÓÖŠµÄ¹²¼Ū¼üŅ»¶ØŹĒ·Ē¼«ŠŌ¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
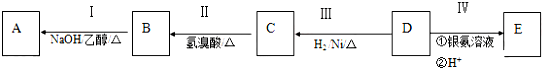
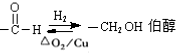

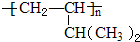
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŌŖ ĖŲ | Mn | Fe | |
| µēĄėÄÜ /kJ•mol-1 | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | 3248 | 2957 | |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com