
����������(����ʽΪC4H2O4Fe���ṹ��ʽΪ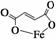 )��һ������ʹ�õ���ǿ������
)��һ������ʹ�õ���ǿ������
(1)��ͼΪʵ����ģ�ҵ��ȡ����������������ͼ��
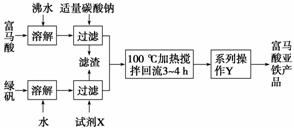
�ٸ������⣬����д��������Ľṹ��ʽ��___________________________________��
���̷�(FeSO4·7H2O)�ڱ���������γɵ�������Ҫ��________(�ѧʽ)��
�۲���Y����________����ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȡ�
���жϲ���Y�С�ϴ�ӡ�������������Ʒ��ϴ����ʵ�鷽����________________________________________________________________________��
(2)���һ��ʵ�鷽����֤�����ø�����������Ʒ����������(�ɹ�ѡ�õ��Լ���KSCN��Һ������KMnO4��Һ��ϡ���ᡣ��֪SCN���ܱ�MnO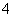 ����)����д�±���Ӧ�ո�
����)����д�±���Ӧ�ո�
| ���� | ʵ����������� |
| �� | ȡ������������Ʒ1.5 g������ϡ����25 mL����ˮϡ����50 mL������ʹ����ȫ�ܽⲢ��Ӧ����ȴ�����(��ȥ���ɵĸ����ἰ���ܹ����ķ�Ӧ��)��������Һ |
| �� | |
| �� | |
| �� |
�𰸡�(1)��HOOCHC===CHCOOH
��Fe(OH)3��Fe2(SO4)3(����������Ҳ��)��������Ũ������ȡ����ϴ��Һ���Թ��У������м������������ữ���ټ����Ȼ�����Һ���ް�ɫ��������
(2)��ȡ������Һ���μ�KSCN��Һ������Ѫ��ɫ
��ȡ������Һ���μӵ���������KMnO4��Һ������Һ��ɫ
����������ɫ�����Һ�еμ�KSCN��Һ����Ѫ��ɫ
������ (1)�����и����ĸ����������Ľṹ��ʽ�е���ԭ�ӻ���������ԭ�ӣ����õ�������Ľṹ��ʽ��HOOCHC===CHCOOH���̷��е����������ڿ��������ױ����������ʣ��������̵Ļ�ѧ����ʽ����д�ɣ�12FeSO4��3O2��6H2O===4Fe(OH)3����4Fe2(SO4)3���ɴ˿ɵó��̷��е����ʿ���ΪFe(OH)3��Fe2(SO4)3������Y�ǴӸ�����������Һ�л�ø�������������Ĺ��̣���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȡ��ж��Ƿ�ϴ���ķ����Ǽ��鸻������������ı����Ƿ��п��������ʣ���õķ����Ǽ���ϴ��Һ���Ƿ����SO
(1)�����и����ĸ����������Ľṹ��ʽ�е���ԭ�ӻ���������ԭ�ӣ����õ�������Ľṹ��ʽ��HOOCHC===CHCOOH���̷��е����������ڿ��������ױ����������ʣ��������̵Ļ�ѧ����ʽ����д�ɣ�12FeSO4��3O2��6H2O===4Fe(OH)3����4Fe2(SO4)3���ɴ˿ɵó��̷��е����ʿ���ΪFe(OH)3��Fe2(SO4)3������Y�ǴӸ�����������Һ�л�ø�������������Ĺ��̣���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȡ��ж��Ƿ�ϴ���ķ����Ǽ��鸻������������ı����Ƿ��п��������ʣ���õķ����Ǽ���ϴ��Һ���Ƿ����SO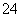 ��
��
(2)��������������ѡ�õ��Լ���ϡ���ᡢKSCN��Һ�����Ը��������Һ������ʱҪ���ų�Fe3���ĸ��ţ������ȼ�KSCN��Һ������Ѫ��ɫ��˵����Fe3����Ȼ����ȡ������Һ���μӵ��������Ը��������Һ�������������ӣ�ע�������ز��ܹ�������������ĸ�����ػ�����SCN����


 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���ӷ�Ӧ��Fe3����3SCN��===Fe(SCN)3�����з�ӦѸ�١��������Ե��ص㣬�Ǽ���Fe3�����õķ���֮һ��ij��ѧ��ȤС��Ϊ̽��Fe(SCN)3�����ʣ���������ʵ�飺
��ȡ10 mL 1 mol/L FeCl3��Һ���μ�3��4��ŨKSCN��Һ������Һ�������Ѫ��ɫ��
��ȡ����Ѫ��ɫ��Һ���μ�����Ũ���ᣬ���ã���Һ��Ϊ��ɫ��ͬʱ���������ĺ���ɫ��������A��
�۽�����������Aͨ�������Ba(OH)2��Һ�У�������ɫ����B��ʣ������C������C��ɫ��ζ����ʹȼ�յ�ľ��Ϩ�𣬿��ŷŵ������У�����ı�����ijɷ֡�
�ܹ��ˣ����ɫ����B�еμ�����ϡ���ᣬ������ȫ�ܽ⣬ͬʱ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ����D��
��ȡ������ɫ��Һ�������μ�BaCl2��Һ������������ϡ����İ�ɫ����E��
��������ʵ�����ش��������⣺
(1)B�Ļ�ѧʽΪ________________��E�Ļ�ѧʽΪ__________��
(2)�������A�ijɷ���________________(�ѧʽ)��
(3)��ȤС��ͬѧ��������ʵ����������ó����ۣ�Fe(SCN)3���л�ԭ�ԣ���Ӧ���б�������Ԫ����________________(��Ԫ�ط���)��
(4)ʵ����У���ԭ���������������ʵ���֮��Ϊ_________��
(5)С��ͬѧ������ʵ���еõ�����������SCN����Ӽ���Fe2��ʱӦע��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ��ѡ��5�л���ѧ����]��15�֣�
�л���AΪ����������������������Է�������Ϊ92��ij����С����AΪ��ʼԭ�Ͽ��Ժϳ���������H�߷��ӻ�����I������ط�Ӧ����ͼ��ʾ��
��֪������Ϣ��
�� ̼ϩ��:CH2���ֳƿ�������ʮ�ֻ�Ծ������������������δ�ɶԵ��Ӳ����������ӵ�C-H��֮��ʹ̼��������
�� ͨ����ͬһ��̼ԭ�����������ǻ����ȶ�������ˮ�γ��ʻ���
�ش��������⣺
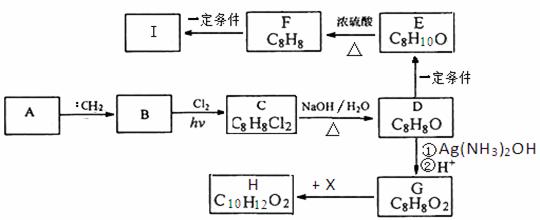
��1��A�Ļ�ѧ����Ϊ ��
��2����B����C�Ļ�ѧ����ʽΪ ���÷�Ӧ����Ϊ �� ��3��G�Ľṹ��ʽΪ ��
��4����д����F����I�Ļ�ѧ����ʽ ��
��5��д��G��H�ķ�Ӧ����ʽ ��
��6��H������ͬ���칹���У��������������Ĺ��� �֣�
 �����
���б���  ������ֻ��һ��ȡ����
������ֻ��һ��ȡ����  ��������
��������
���к˴Ź������������ֲ�ͬ��ѧ�������⣬�ҷ������Ϊ1:1:2:2:6����
��д�ṹ��ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��X��Ϊ��ѧ�����Ĵ��������֮��������ת����ϵ(����������ȥ)��
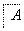
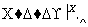
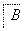
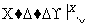
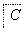
(1)��X��ǿ���������嵥�ʣ���A������______(�����)��
a��C b��Al c��Na d��Mg
(2)��X�ǽ������ʣ���C��ˮ��Һ�еμ�AgNO3��Һ������������ϡHNO3�İ�ɫ������X��A��ȼ�ղ����ػ�ɫ���̡�B�Ļ�ѧʽΪ________��C��Һ������ʱӦ��������X��������(�ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ)______ __�������C��Һ�н���Ԫ�صIJ���������
__�������C��Һ�н���Ԫ�صIJ��������� ________
________ ________����C����Һ�м�������������Һ��������__________����
________����C����Һ�м�������������Һ��������__________���� д���˹���������������ԭ��Ӧ�Ļ�ѧ����ʽ__________________��
д���˹���������������ԭ��Ӧ�Ļ�ѧ����ʽ__________________��
(3)��A��B��C��Ϊ����ͬ�ֽ���Ԫ�صĻ����X��ǿ���ǿ���B�Ļ�ѧʽΪ__________����Ӧ�ٵ����ӷ���ʽΪ______________________��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ж����е������Һ�е�������Ŀ
(1)��0.4 mol Al2(SO4)3����Һ�У���________mol SO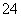 ��Al3�������ʵ���________
��Al3�������ʵ���________
0.8 mol(�>������<������)��
(2)1 L 0.1 mol·L��1��CH3COOH��Һ�У�n(CH3COOH)________ 0.1 mol��
n(CH3COO��)________ 0.1 mol��n(H2O)________ 0.1 mol(����ڡ�����С�ڡ��� �ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͬ��ͬѹ�£�x g�������y g������ռ����ͬ����������ݰ����ӵ������ж���������������� (����)
A��x��y���ڼ����ҵ���Է�������֮��
B��x��y���ڼ����ҵķ��Ӹ���֮��
C��x��y����ͬ��ͬѹ�¼����ҵ��ܶ�֮��
D��y��x����ͬ��ͬ����£��������ļ����ҵ�ѹǿ֮��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA���������ӵ���������ֵ������˵������ȷ���� (����)
�ٳ��³�ѹ�£�22.4 L N2�ķ�����ΪNA����64 gͭ����������ԭ��Ӧ��һ��ʧȥ2NA�����ӡ��۳��³�ѹ�£�100 mL 0.5 mol·L��1�Ĵ�����Һ�У�����ķ�����ĿС��0.05NA���ܳ��³�ѹ�£�1 mol�������еĺ��������Ϊ2NA
A���٢� B���ۢ� C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������������������ŵĶ�Ӧ��ϵ����ȷ����
A��CH3CH=CH2�� ϩ�� 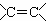 B��
B��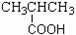 ���ᡡ��COOH
���ᡡ��COOH
C��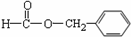 ȩ�ࡡ��CHO D��CH3��NH2 ���� ��NH2
ȩ�ࡡ��CHO D��CH3��NH2 ���� ��NH2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com