
���� ��1������n=$\frac{N}{{N}_{A}}$����ag��������ʵ���������֮�ȵ��������ʵ���֮�ȣ��ݴ˼���cg����������ʵ���������V=n��Vm�����������
��2�����ȷ���Һ��һ�ݼ�BaCl2����Һ����Ba2++SO42-�TBaSO4�����ɷ���ʽ��֪n��SO42-��=n��Ba2+������һ�ݼ�����������Һʱ����Mg2++2OH-�TMg��OH��2�����ɷ���ʽ��֪ÿ����Һ��n��Mg2+��=$\frac{1}{2}$n��OH-����
�����õ���غ��֪ÿ����2n��Mg2+��+n��K+��=2n��SO42-�����ݴ˼���ÿ����n��K+��������c=$\frac{n}{V}$���������Ũ�ȣ�
��3���������������֪����14.2g��ˮNa2SO4����180gˮ�����γɱ�����Һ��
�赱�����Һ�м���xgNa2SO4•10H2Oǡ���γɱ�����Һ��������xgNa2SO4•10H2O�к��е�����Na2SO4������Ϊx��$\frac{142}{322}$g�������ˮ������Ϊx��$\frac{180}{322}$g�����ܼ�ˮ������Ϊ��180+x��$\frac{180}{322}$��g�����ݱ������㼴�ɣ�
��� �⣺��1��a��ij�����к��еķ�����Ϊb����c�����庬�еķ�����Ϊ$\frac{cb}{a}$��c�˸���������ʵ���Ϊ$\frac{\frac{bc}{a}}{N{\;}_{A}mol{\;}^{-1}}$=
$\frac{bc}{a{N}_{A}}$mol���ڱ�״����Vm=22.4L/mol����cg����������Ϊ$\frac{bc}{a{N}_{A}}$mol��22.4L/mol=$\frac{22.4bc}{a{N}_{A}}$L��
�ʴ�Ϊ��$\frac{22.4bc}{a{N}_{A}}$��
��2�������Һ�ֳ����ȷݣ�ÿ����ҺŨ����ԭ��ҺŨ����ͬ��
һ�ݼ�BaCl2����Һ����Ba2++SO42-�TBaSO4�����ɷ���ʽ��֪n��SO42-��=n��Ba2+��=n��BaCl2��=bmol��
��һ�ݼ�����������Һʱ����Mg2++2OH-�TMg��OH��2�����ɷ���ʽ��֪ÿ����Һ��n��Mg2+��=$\frac{1}{2}$n��OH-��=$\frac{1}{2}$amol��
�ɵ���غ��֪ÿ����2n��Mg2+��+n��K+��=2n��SO42-������ÿ������Һn��K+��=2bmol-2��$\frac{1}{2}$amol=��2b-a��mol����ԭ��Һ�м�����Ũ��=$\frac{��2b-a��mol}{\frac{V}{2}L}$=$\frac{2��2b-a��}{V}$mol/L��
�ʴ�Ϊ��$\frac{2��2b-a��}{V}$��
��3���������������֪����14.2g��ˮNa2SO4����180gˮ�����γɱ�����Һ��
�赱�����Һ�м���xgNa2SO4•10H2Oǡ���γɱ�����Һ�����ڼ����xgNa2SO4•10H2O�к��е�����Na2SO4������Ϊx��$\frac{142}{322}$g=$\frac{142x}{322}$g���ᾧˮ����ˮ��Ҳ��Ϊ�ܼ����������ˮ������Ϊx��$\frac{180}{322}$g=$\frac{180x}{322}$g�����ܼ�ˮ������Ϊ��180+$\frac{180x}{322}$��g�����У�14.2g��180g
$\frac{142x}{322}$g����180+$\frac{180x}{322}$��g
���У�$\frac{\frac{14.2g}{142xg}}{322}$=$\frac{180g}{��\frac{180x}{322}+180��g}$����ã����x=35.8g��
�ʴ�Ϊ��35.8g��
���� ���⿼�������ʵ����йؼ��㣬��ȷ�����ʵ���Ϊ���ļ��㹫ʽ����Ϥ���ʵ���Ũ�ȸ����ǽ���ؼ������б�����Һ���йؼ��㣬Ӧע���������ᾧˮ�����ʵ�ʱ�ᾧˮ����ˮ��Ҳ��Ϊ�ܼ����Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ��Ŀ�� | ʵ����� | |
| A | �Ƚ�ˮ���Ҵ�����Ļ����� | �ֱ�������Ͷ�뵽ʢ��ˮ���Ҵ����ձ��� |
| B | ��MgCl2��Һ�Ʊ���ˮMgCl2 | ��MgCl2��Һ�������� |
| C | ��ȥCu���л��е�CuO | ����ϡ������Һ�����ˡ�ϴ�ӡ����� |
| D | �Ʊ�Fe��OH��3���� | ��NaOHŨ��Һ�μӵ����͵�FeCl3��Һ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȼ�����Һ��ͭ��Ӧ��Fe3++Cu�TFe2++Cu2+ | |
| B�� | ��������ϡ���ᷴӦ��Na2SiO3+2H+�T2Na++H2SiO3�� | |
| C�� | ������ˮ��Ӧ��Cl2+H2O�T2H++Cl-+ClO- | |
| D�� | ������þ��ϡ���ᷴӦ��Mg��OH��2+2H+�TMg2++2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
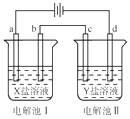 ��ͼ��ʾ�ĵ��آ�͢��У�a��b��c��d��ΪPt�缫���������У��缫b��d��û�������ݳ���������������������b��d����������ʵ����������Һ�ǣ�������
��ͼ��ʾ�ĵ��آ�͢��У�a��b��c��d��ΪPt�缫���������У��缫b��d��û�������ݳ���������������������b��d����������ʵ����������Һ�ǣ�������| ѡ�� | X | Y |
| A | MgSO4 | CuSO4 |
| B | CuSO4 | AgNO3 |
| C | FeSO4 | Al2��SO4��3 |
| D | AgNO3 | Pb��NO3��2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 150mL1mol•L-1NaCl��Һ | B�� | 75mL1mol•L-1CaCl2��Һ | ||
| C�� | 150mL1mol•L-1KCl��Һ | D�� | 75mL1mol•L-1AlCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol���ǿ���ˮ������2mol������ | |
| B�� | �Ҵ�������ȥ��Ӧ���Ƶ���ϩ | |
| C�� | ��CH3��2CHCH��CH3��2��������2��3-������ | |
| D�� | �ޣ��飬˿��ëȼ�պ�ֻ����CO2��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��� | B�� | ʯ��ʯ | C�� | ��ʯ | D�� | ʯӢ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | Al3+��Fe2+��Ba2+��Na+��NH4+ |
| ������ | Cl-��CO32-��SO42-��NO3-��I- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com