
�Ĵ���֦���̲طḻ�ķ����ѡ�����Դ����������������Ҫ�ɷ�ΪTiO2��FeO��Fe2O3,Ti������ϼ�Ϊ+4����ԭ�ϣ�������ɫ���϶������ѵ���Ҫ�������£�
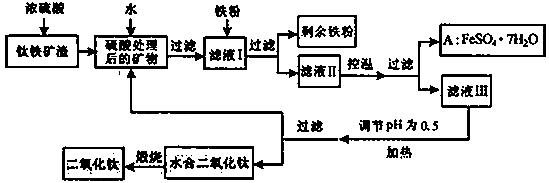
��ش��������⣺
������������ѷ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
����ҺI�м������ۣ�������Ӧ�����ӷ���ʽΪ��_________________________��_______________________________________��
��ʵ�����������У����ˮ�м�����Һ��ʹ���ҺpH��0.5�����ο�ʼˮ�⡣ˮ������в���ͨ�����ˮ������ά����Һ����һ��ʱ�䣬���γ��ˮ������ˮ�϶������ѳ�����������ѧ��ѧƽ��ԭ������ͨ�����ˮ���������ã�_______________________________________________��
���˷����ˮ�϶������ѳ�������Һ���ص���ҪĿ���dz��������Һ�е����Ρ�___________��______________��_______________________���ѧʽ�������ٷ����ŷš�
��4��A������������ɫ���ϣ�Fe2O3��,�䷽���ǣ���556a kgA��Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���3336b kg A��112c kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����_______________________kg��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
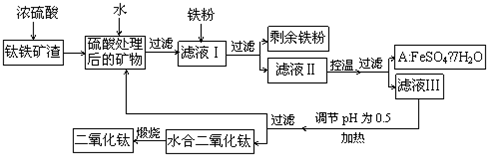
�Ĵ���֦���̲طḻ�ķ����ѡ�����Դ����������������Ҫ�ɷ�ΪTiO2��FeO��Fe2O3,Ti������ϼ�Ϊ+4����ԭ�ϣ�������ɫ���϶������ѵ���Ҫ�������£�![]()
![]()
![]() w_w w. k#s5_u.c o*m
w_w w. k#s5_u.c o*m

��ش��������⣺![]()
![]()
![]() w_w w. k#s5_u.c o*m
w_w w. k#s5_u.c o*m
��1�� ������������ѷ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
��2�� ����ҺI�м������ۣ�������Ӧ�����ӷ���ʽΪ��_________________________��_______________________________________��
��3�� ��ʵ�����������У����ˮ�м�����Һ��ʹ���ҺpH��0.5�����ο�ʼˮ�⡣ˮ������в���ͨ�����ˮ������ά����Һ����һ��ʱ�䣬���γ��ˮ������ˮ�϶������ѳ�����������ѧ��ѧƽ��ԭ������ͨ�����ˮ���������ã�_______________________________________________��
���˷����ˮ�϶������ѳ�������Һ���ص���ҪĿ���dz��������Һ�е����Ρ�___________��______________��_______________________���ѧʽ�������ٷ����ŷš�
��4��A������������ɫ���ϣ�Fe2O3��,�䷽���ǣ���556a kgA��Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���3336b kg A��112c kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����_______________________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ĵ���֦���̲طḻ�ķ����ѡ�����Դ����������������Ҫ�ɷ�ΪTiO2��FeO��Fe2O3,Ti������ϼ�Ϊ+4����ԭ�ϣ�������ɫ���϶������ѵ���Ҫ�������£�

��ش��������⣺
������������ѷ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
����ҺI�м������ۣ�������Ӧ�����ӷ���ʽΪ��_________________________��_______________________________________��
(3)��ʵ�����������У����ˮ�м�����Һ��ʹ���ҺpH��0.5�����ο�ʼˮ�⡣ˮ������в���ͨ�����ˮ������ά����Һ����һ��ʱ�䣬���γ��ˮ������ˮ�϶������ѳ�����������ѧ��ѧƽ��ԭ������ͨ�����ˮ���������ã�_______________________________________________��
���˷����ˮ�϶������ѳ�������Һ���ص���ҪĿ���dz��������Һ�е����Ρ�___________��______________��_______________________���ѧʽ�������ٷ����ŷš�
��4��A������������ɫ���ϣ�Fe2O3��,�䷽���ǣ���556a kg A��Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���3336b kg A��112c kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����_______________________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ��ɽ�и�����ѧģ���Ծ����ˣ� ���ͣ������
��16�֣��Ĵ���֦���̲طḻ�ķ����ѡ�����Դ����������������Ҫ�ɷ�ΪTiO2��FeO��Fe2O3,Ti������ϼ�Ϊ+4����ԭ�ϣ�������ɫ���϶������ѵ���Ҫ�������£�

��ش��������⣺

��1�� ������������ѷ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
��2�� ����ҺI�м������ۣ�������Ӧ�����ӷ���ʽΪ��_________________________��_______________________________________��
��3�� ��ʵ�����������У����ˮ�м�����Һ��ʹ���ҺpH��0.5�����ο�ʼˮ�⡣ˮ������в���ͨ�����ˮ������ά����Һ����һ��ʱ�䣬���γ��ˮ������ˮ�϶������ѳ�����������ѧ��ѧƽ��ԭ������ͨ�����ˮ���������ã�_______________________________________________��
���˷����ˮ�϶������ѳ�������Һ���ص���ҪĿ���dz��������Һ�е����Ρ�___________��______________��_______________________���ѧʽ�������ٷ����ŷš�
��4��A������������ɫ���ϣ�Fe2O3��,�䷽���ǣ���556a kgA��Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���3336b kg A��112c kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����_______________________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ĸ����������¿��������ۺϣ���ѧ�� ���ͣ������
�Ĵ���֦���̲طḻ�ķ����ѡ�����Դ����������������Ҫ�ɷ�ΪTiO2��FeO��Fe2O3,Ti������ϼ�Ϊ+4����ԭ�ϣ�������ɫ���϶������ѵ���Ҫ�������£�

��ش��������⣺
(1)������������ѷ�Ӧ�Ļ�ѧ����ʽ��____________________________��2�֣�
(2)����ҺI�м������ۣ�������Ӧ�����ӷ���ʽΪ��______________________��
________________________________������2�֣�
(3)��ʵ�����������У����ˮ�м�����Һ��ʹ���ҺpH��0.5�����ο�ʼˮ�⡣ˮ������в���ͨ�����ˮ������ά����Һ����һ��ʱ�䣬���γ��ˮ������ˮ�϶������ѳ�����������ѧ��ѧƽ��ԭ������ͨ�����ˮ���������ã�__________________________��2�֣�
(4)���˷����ˮ�϶������ѳ�������Һ���ص���ҪĿ���dz��������Һ�е����Ρ�___________��_____________��___________________���ѧʽ����2�֣������ٷ����ŷš�
(5)A������������ɫ���ϣ�Fe2O3��,�䷽���ǣ���556a kgA��Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���3336b kg A��112c kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����_____________kg��1�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com