
��֪Cl2ͨ��Ũ��ˮ�лᷢ�����·�Ӧ��3Cl2��8NH2![]() 6NH4Cl��N2���������Ϊ11.2L������Ϊ3.335g��Cl2��N2�Ļ������ͨ��Ũ��ˮ�������Ϊ0.672L������Cl2��N2��ռ50��(�������)]��(����������ڱ�״���²ⶨ)
6NH4Cl��N2���������Ϊ11.2L������Ϊ3.335g��Cl2��N2�Ļ������ͨ��Ũ��ˮ�������Ϊ0.672L������Cl2��N2��ռ50��(�������)]��(����������ڱ�״���²ⶨ)
(1)�����㣬�������İ�������Ϊ________g��(����д���������)
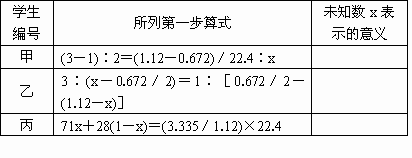
(2)��������λѧ���Ӳ�ͬ�ǶȽ������ʱ���еĵ�һ����ʽ�����ж���������δ֪��x�ֱ��ʾʲô��������д�ڱ����ڣ�(Ϊ��㣬��λδ�г�)
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ||
| ||
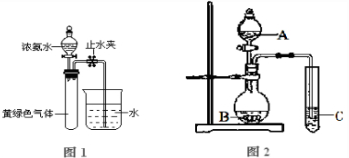
 �ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
�ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���������еھ���ѧ2006-2007ѧ�����ѧ�ڸ����꼶�ڶ����¿�����ѧ�Ծ� ���ͣ�038
| |||||||||||
�鿴�𰸺ͽ���>>
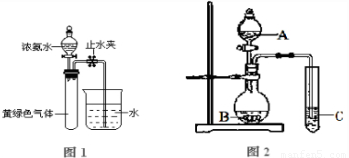
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʦ���и������ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com