
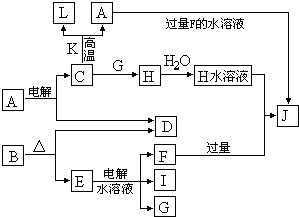
��15�֣�A��G��J��Ϊ�ǽ������ʣ���ҵ�����÷�Ӧ����ұ����Aͬ����Ԫ��G���ʵĴֲ�Ʒ��KΪ��ɫҺ�壬D��һ�ֳ�������������ij�������еõ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��

��1��д���������ʵĻ�ѧʽB______�� F_______��H______��A��G�������̬�⻯���У����ȶ��������� ���ѧʽ����д����Ӧ�ٵĻ�ѧ����ʽ ��
��2����Ӧ���ǹ�ҵ��ұ������D�ķ���֮һ��Ȼ��Ŀǰ������60%��D�����ǴӺ�ˮ����ȡ�ġ������Ǻ�ˮ����������D�ļ�������ͼ����Ϲ�ҵ����ʵ�ʣ�����������д��Ҫ�������ʵĻ�ѧʽ����������д�������ʵĻ�ѧʽ��

��3�������к���H�������ӣ����ù������⡢ϡ����͵����������������ӣ�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��4��д��G��J�Ļ�ѧ����ʽ________________________________________��ijͬѧ������ij����������ƽ��G��J��ϵ�������й�ϵ���ϴ˼�������ȷ����_________
A����Ӧ�٣�F:G=1:1 B����Ӧ�ڣ�A:D=1:1
C����Ӧ�ۣ�A:J=2:1 D����Ӧ�ܣ�E:B =1:1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
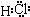
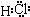
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C��D��G��I��Ϊ������Ԫ���γɵĵ��ʣ�D��G��IΪ�����ǽ�����̬���ʡ�DԪ�ص�ԭ�ӵ������������Ǵ�����������3����C��Gͬ���ڣ���ԭ���������������4�����ǵļ����ӵ��Ӳ�ṹ��ͬ�����������ת����ϵ��
����գ�
(1) D��I���γ�ԭ�Ӹ�����Ϊ1��1�Ĺ��ۻ������д�������ʽ�� ��
��2��L��ĿǰӦ����㷺�Ľ�������̼����������L��������д�����Eˮ��Һ�Ļ�ѧʽ�� ��
��3��������K �к�����ɵ���L��Ԫ�أ��Ҹ�Ԫ�ص���������Ϊ70%����Ӧ�ٵĻ�ѧ����ʽ�� �������÷�Ӧ�IJ�����
��4��д��A+F ��J�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�������ʡ�����и���������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��15�֣�A��G��J��Ϊ�ǽ������ʣ���ҵ�����÷�Ӧ����ұ����Aͬ����Ԫ��G���ʵĴֲ�Ʒ��KΪ��ɫҺ�壬D��һ�ֳ�������������ij�������� �õ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��
�õ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��
��1��д���������ʵĻ�ѧʽB______�� F_______�� H______��A��G�������̬�⻯���У����ȶ��������� ���ѧʽ����д����Ӧ�ٵĻ�ѧ����ʽ ��
��2����Ӧ���ǹ�ҵ��ұ������D�ķ���֮һ��Ȼ��Ŀǰ������60%��D�����ǴӺ�ˮ����ȡ�ġ������Ǻ�ˮ����������D�ļ�������ͼ����Ϲ�ҵ����ʵ�ʣ�����������д��Ҫ�������ʵĻ�ѧʽ����������д�������ʵĻ�ѧʽ��
��3�������к���H�������ӣ����ù������⡢ϡ����͵����������������ӣ�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��4��д��G��J�Ļ�ѧ����ʽ________________________________________��ijͬѧ������ij����������ƽ��G��J��ϵ�������й�ϵ���ϴ˼�������ȷ����_________
A����Ӧ�٣�F:G="1:1 " B����Ӧ�ڣ�A:D=1:1
C����Ӧ�ۣ�A:J="2:1 " D����Ӧ�ܣ�E:B =1:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������ʡ����������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��15�֣�A��G��J��Ϊ�ǽ������ʣ���ҵ�����÷�Ӧ����ұ����Aͬ����Ԫ��G���ʵĴֲ�Ʒ��KΪ��ɫҺ�壬D��һ�ֳ�������������ij�������еõ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��
��1��д���������ʵĻ�ѧʽB______�� F_______�� H______��A��G�������̬�⻯���У����ȶ��������� ���ѧʽ����д����Ӧ�ٵĻ�ѧ����ʽ ��
��2����Ӧ���ǹ�ҵ��ұ������D�ķ���֮һ��Ȼ��Ŀǰ������60%��D�����ǴӺ�ˮ����ȡ�ġ������Ǻ�ˮ����������D�ļ�������ͼ����Ϲ�ҵ����ʵ�ʣ�����������д��Ҫ�������ʵĻ�ѧʽ����������д�������ʵĻ�ѧʽ��

��3�������к���H�������ӣ����ù������⡢ϡ����͵����������������ӣ�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��4��д��G��J�Ļ�ѧ����ʽ________________________________________��ijͬѧ������ij����������ƽ��G��J��ϵ�������й�ϵ���ϴ˼�������ȷ����_________
A����Ӧ�٣�F:G=1:1 B����Ӧ�ڣ�A:D=1:1
C����Ӧ�ۣ�A:J=2:1 D����Ӧ�ܣ�E:B =1:1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com