
ŹµŃéÄæµÄ£ŗĢ½¾æ¹żŃõ»ÆÄĘÓėĖ®·“Ó¦ŗóµÄČÜŅŗµĪ¼Ó·ÓĢŖŹŌŅŗĻȱäŗģŗóĶĖÉ«µÄŌŅņ”£
[·ÖĪöÓė²ĀĻė]
£Ø1£©øł¾Ż¹żŃõ»ÆÄĘÓėĖ®·“Ó¦µÄŌĄķ£ŗ2Na2O2 + 2H2O =" 4NaOH" + O2”ü£¬Ķł¹żŃõ»ÆÄĘ¹ĢĢåĶźČ«Čܽā·“Ó¦ŗóµÄČÜŅŗÖŠµĪ¼Ó·ÓĢŖ±¾Ó¦Ö»»į±äŗģ¶ų²»»įĶĖÉ«£¬¶ųŹµŃéÖŠ·¢ĻÖ·ÓĢŖ±äŗģŗóÓÖĶĖÉ«”£ÓÉ“ĖĢį³öČēĻĀµÄ²ĀĻė£ŗ
A£®ŃõĘųÓŠĘư׊Ō
B£®ĒāŃõ»ÆÄĘÓŠĘư׊Ō
C£®
[ŹµŃéÓėÅŠ¶Ļ] ĒėĶź³ÉĻĀĮŠ±ķøń£ŗ
| ŹµŃ鱹ŗÅ | 1 | 2 | 3 |
| ŹµŃé×°ÖĆ | 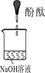 | 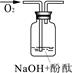 | 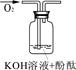 |
| ŃéÖ¤²ĀĻė | | C | |
| ŹµŃéĻÖĻó | ČÜŅŗ±äŗģŗó²»ĶĖÉ« | ||
| ŹµŃéĖµĆ÷ | 1”¢2µÄŹµŃéÖŠNaOHČÜŅŗŹĒÓĆ £ØĢī”°ĒāŃõ»ÆÄĘ¹ĢĢå”±”¢”°Ńõ»ÆÄĘ¹ĢĢå”±”¢”°¹żŃõ»ÆÄĘ¹ĢĢå”±£©ČÜÓŚĖ®ÅäÖʵĔ£ | ||
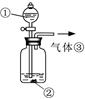
£Ø1£©ŃõĘųŗĶĒāŃõ»ÆÄĘ¹²Ķ¬×÷ÓĆ¾ßÓŠĘư׊Ō B A ĒāŃõ»ÆÄĘ¹ĢĢå£Øø÷1·Ö¹²3·Ö£©
£Ø2£©H2O2
£Ø3£©B C £Øø÷1·Ö£©
£Ø4£©O2£Ø1·Ö£© H2O2²»ĪČ¶Ø£¬ŅŃ·Ö½ā
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©½įŗĻA”¢B£¬CµÄ²ĀĻėŹĒŃõĘųŗĶĒāŃõ»ÆÄĘ¹²Ķ¬×÷ÓĆ¾ßÓŠĘư׊Ō£¬ĖłŅŌŹµŃé1¶ŌÓ¦µÄŹĒB£¬ŹµŃé3¶ŌÓ¦µÄŹĒA£»ĒāŃõ»ÆÄĘČÜŅŗŹĒÓĆĒāŃõ»ÆÄĘ¹ĢĢåÅäÖĘµÄ£¬ŅņĪŖŃõ»ÆÄĘ¹ĢĢåæÕĘųÖŠŅ×±äÖŹ£¬Ōģ³ÉĖłÅäČÜŅŗ ²»“棬¹żŃõ»ÆÄĘ¹ĢĢåÓėĖ®×÷ÓĆ³żÉś³ÉĒāŃõ»ÆÄĘĶā»¹»įÓŠŃõĘųÉś³É£¬øųŹµŃé“ųĄ“øÉČÅ
£Ø2£©ÄĘŌŖĖŲŠĪ³ÉĮĖĪČ¶ØµÄ»ÆŗĻĪļ¼“ĒāŃõ»ÆÄĘ£¬ĮķŅ»ÖÖ²»ŗÜĪČ¶Ø”¢¾ßÓŠĘư׊ŌµÄĪļÖŹXÓ¦ŹĒ¹żŃõ»ÆĒā£¬ Ęä»ÆѧŹ½ĪŖH2O2
£Ø3£©¹żŃõ»ÆĒāŌŚ¶žŃõ»ÆĆĢ×÷“߻ƼĮµÄĢõ¼žĻĀ·Ö½ā²śÉśŃõĘų£¬ŃõĘų æÉÓĆ“ų»šŠĒµÄľĢõ¼ģŃ飬ĖłŅŌ¢Ł“¦×°
ČėµÄĪļÖŹ¹żŃõ»ÆÄĘÓėĖ®·“Ó¦ŗóµÄČÜŅŗ£¬Ń”B£¬¢Ś“¦×°ČėµÄĪļÖŹŹĒ¶žŃõ»ÆĆĢ£¬Ń”C”£
£Ø4£©ĘųĢå¢ŪŹĒŃõĘų£¬ŅņĪŖ¹żŃõ»ÆĒā²»ĪČ¶Ø£¬·Ö½ā²śÉśŃõĘų£¬ĖłŅŌŌŚŹéŠ“¹żŃõ»ÆÄĘÓėĖ®µÄ·“Ó¦·½³ĢŹ½Ź±²»Š“³ö
æ¼µć£ŗæ¼²é¶ŌŹµŃéµÄ²ĀĻė”¢·ÖĪöÅŠ¶ĻÄÜĮ¦£¬¹żŃõ»ÆÄĘÓėĖ®·“Ó¦ŌĄķµÄ·ÖĪö



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ŅŃÖŖA”«GÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·ÖÉś³ÉĪļŅŃĀŌČ„£©£¬ĘäÖŠA”¢GĪŖµ„ÖŹ£¬DŹĒÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶É«µÄĘųĢ壬E”¢F¾łÄÜÓėNaOHČÜŅŗ·“Ó¦”£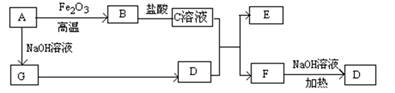
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öFµÄµē×ÓŹ½ £»
£Ø2£©¢ŁCČÜŅŗÓėD·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»
¢ŚFČÜŅŗÓėNaOHČÜ???¹²ČČ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
£Ø3£©¢ŁĒėÓĆĄė×Ó·½³ĢŹ½½āŹĶCČÜŅŗĪŖŗĪĻŌĖįŠŌ £»
¢ŚFČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ £»
£Ø4£©½«5.4gAĶ¶Čė200mL 2.0mol/LijČÜŅŗÖŠÓŠGµ„ÖŹ²śÉś£¬ĒŅ³ä·Ö·“Ó¦ŗóÓŠ½šŹōŹ£Óą£¬ŌņøĆČÜŅŗæÉÄÜŹĒ £ØĢī“śŗÅ£©
A£®HNO3ČÜŅŗ B£®H2SO4ČÜŅŗ C£®NaOHČÜŅŗ D£®HClČÜŅŗ
£Ø5£©½«1molN2ŗĶ3molG¼°“߻ƼĮ³äČėČŻ»żĪŖ2LµÄijĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£¬ŅŃÖŖøĆ·“Ó¦ĪŖ·ÅČČ·“Ó¦”£Ę½ŗāŹ±£¬²āµĆDµÄĪļÖŹµÄĮæÅضČĪŖa mol/L”£
¢ŁČē¹ū·“Ó¦ĖŁĀŹv(G)£½1.2mol/(L”¤min)£¬Ōņv(D)£½ mol/(L”¤min)
¢ŚŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ČōĘšŹ¼Ź±³äČė0.5molN2ŗĶ1.5molG“ļµ½Ę½ŗāŗó£¬DµÄĪļÖŹµÄĮæÅØ¶Č £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©a/2 mol/L”£
¢ŪøĆĢõ¼žĻĀµÄĘ½ŗā³£ŹżĪŖ £ØÓĆŗ¬aµÄ“śŹżŹ½±ķŹ¾£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Ä³Ń§ÉśÓĆŹµŃéŹŅ³£¼ūµÄĖį”¢¼ī”¢ŃĪŗĶ½šŹōµ„ÖŹĪŖ·“Ó¦Īļ£¬²¢ĄūÓĆŅ»øöµ×²æÓŠŠ”æ׵ďŌ¹ÜŗĶŅ»øö¹ćæŚĘæ×é×°³ÉČēĶ¼ĖłŹ¾µÄ×°ÖĆ”£ŹŌ»Ų“š£ŗ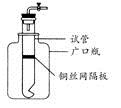
(1)ČōŹŌ¹Ü֊װӊĶĖæĶųøō°å£¬ĄūÓĆøĆ×°ÖĆæÉÖĘČ”ÄÄŠ©ĘųĢå£æ
(Š“³öĮ½ÖÖ)”£
(2)Čō½«ĶĖæĶųøō°åøÄĪŖĢśĖæĶųøō°å£¬ŌņøĆ×°ÖĆæÉÓĆÓŚÖĘČ”ŗĪÖÖĘųĢå£æ
ӣ
øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŅ¹ś»Æ¹¤×ؼŅŗīµĀ°ń£¬ÓĀÓŚ““ŠĀ£¬øĽų°±¼ī·ØÉč¼ĘĮĖ”°ĮŖŗĻÖĘ¼ī·Ø”±£¬ĪŖŹĄ½ēÖĘ¼ī¹¤Ņµ×÷³öĮĖĶ»³ö¹±Ļ×”£ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©”°ĮŖŗĻÖĘ¼ī·Ø”±ÖʵƵĔ°¼ī”±ŹĒ (Ģī»ÆѧŹ½)”£
£Ø2£©°±¼ī·ØŗĶĮŖŗĻÖĘ¼ī·ØŹĒĮ½“óÖŲŅŖµÄ¹¤ŅµÖĘ¼ī·Ø£¬ĻĀĮŠ±ķ“ļÖŠ£¬²»ÕżČ·µÄŹĒ ”£
| | | °±¼ī·Ø | ĮŖŗĻÖĘ¼ī·Ø |
| A | ŌĮĻ | Ź³ŃĪ”¢°±Ęų”¢ÉśŹÆ»Ņ | Ź³ŃĪ”¢°±Ęų”¢¶žŃõ»ÆĢ¼ |
| B | æÉÄܵÄø±²śĪļ | ĀČ»ÆøĘ | ĀČ»Æļ§ |
| C | Ń»·ĪļÖŹ | °±Ęų”¢¶žŃõ»ÆĢ¼ | ĀČ»ÆÄĘ |
| D | ĘĄ¼Ū | ŌĮĻŅ×µĆ£»Éč±øø“ŌÓ£»ÄÜŗÄøß | ŌĮĻĄūÓĆĀŹøߣ»·ĻĘśĪļÉŁ |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĮņĖįŃĒĢś¾§Ģå(FeSO4”¤7H2O)ŌŚŅ½Ņ©ÉĻ×÷²¹ŃŖ¼Į”£Ä³æĪĶāŠ”×éµÄĶ¬Ń§Óū²ā¶ØøĆ²¹ŃŖ¼ĮÖŠ
ĢśŌŖĖŲµÄŗ¬Į攣ŹµŃé²½ÖčČēĻĀ£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Ö¤Ć÷²½Öč¢ŁĀĖŅŗÖŠŗ¬ÓŠFe2£«µÄ·½·ØŹĒ£ŗȔѳ£¬ĻȵĪ¼ÓKSCNČÜŅŗ£¬ŌŁµĪ¼Ó________£¬øĆ¹ż³ĢµÄĻÖĻóĪŖ________”£
(2)²½Öč¢Ś¼ÓČė¹żĮæH2O2µÄÄæµÄŹĒ£ŗ______________________________________”£
(3)²½Öč¢ŪÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ£ŗ_______________________________________”£
(4)²½Öč¢ÜÖŠŅ»ĻµĮŠ“¦ĄķµÄ²Ł×÷²½Öč£ŗ¹żĀĖ”¢________”¢×ĘÉÕ”¢________”¢³ĘĮ攣
(5)ČōŹµŃéÖŠĢśĪŽĖšŗÄ£¬ŌņĆæʬ²¹ŃŖ¼ĮÖŠŗ¬ĢśŌŖĖŲµÄÖŹĮæĪŖ________g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĀĢ·ÆŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£
”¾ĪŹĢā1”æĀĢ·Æ¾§Ģå£ØFeSO4”¤7H2O£©ÓÉÓŚ±£“ę²»Ķ×»ņ³¤¾Ć·ÅÖĆ£¬ČŻŅ×±»æÕĘųÖŠµÄŃõĘųŃõ»Æ¶ų±äÖŹ”£ĪŖĢ½¾æĀĢ·ÆѳʷµÄ±äÖŹĒéæö£¬Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§Éč¼ĘĮĖĻĀĮŠŹµŃé·½°ø”£
ŹµŃé×¼±ø£ŗȔɣĮæĀĢ·Æѳʷ£¬ÓĆÕōĮóĖ®ŌŚÉÕ±ÖŠÅä³ÉČÜŅŗ”£
£Ø1£©·½°ø1£ŗȔɣĮæČÜŅŗ£¬¼ÓČė¼øµĪ ŹŌ¼Į£ØŠ“»ÆѧŹ½£©£¬Čē¹ū¹Ū²ģµ½µÄĻÖĻóŹĒČÜŅŗ±äŃŖŗģÉ«£»ŹµŃé½įĀŪ£ŗÖ¤Ć÷ĀĢ·ÆѳʷŅѱ»Ńõ»Æ”£
£Ø2£©·½°ø2£ŗȔɣĮæČÜŅŗ£¬¼ÓČė¼øµĪĖįŠŌKMnO4ČÜŅŗ£¬Čē¹ū¹Ū²ģµ½µÄĻÖĻóŹĒ ”£ŹµŃé½įĀŪ£ŗÖ¤Ć÷ĀĢ·ÆѳʷĶźČ«±»Ńõ»Æ”£
£Ø3£©Ź¹ÓĆFeSO4Ź±£¬ČēŅŖ·ĄÖ¹Fe3+µÄøÉČÅ£¬æÉŅŌ¼ÓČėŹŹĮæĢś·Ū½ųŠŠ³żŌÓ£¬Š“³ö¼ÓČėĢś·Ūŗó·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
£Ø4£©ŅŖ“ÓFeSO4ČÜŅŗÖŠµĆµ½ĀĢ·Æ¾§Ģ壬±ŲŠė½ųŠŠµÄŹµŃé²Ł×÷²½Öč£ŗ”””””””””””¢ĄäČ“½į¾§”¢””¹żĀĖ”¢×ŌČ»øÉŌļ£¬ŌŚÕāŅ»ĻµĮŠ²Ł×÷֊ƻӊÓƵ½µÄŅĒĘ÷ÓŠ””””””””””””£Ø“ĖæÕĢīŠņŗÅ£©
| A£®Õō·¢Ćó | B£®ŹÆĆŽĶų | C£®ÉÕ± | D£®²£Į§°ō |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ÓĆŗ¬ÓŠAl2O3”¢SiO2ŗĶÉŁĮæFeO”¤xFe2O3µÄĀĮ»ŅÖʱøAl2(SO4)3”¤18H2O£¬¹¤ŅÕĮ÷³ĢČēĻĀ(²æ·Ö²Ł×÷ŗĶĢõ¼žĀŌ)£ŗ
¢ń.ĻņĀĮ»ŅÖŠ¼ÓČė¹żĮæĻ”H2SO4£¬¹żĀĖ£»
¢ņ.ĻņĀĖŅŗÖŠ¼ÓČė¹żĮæKMnO4ČÜŅŗ£¬µ÷½ŚČÜŅŗµÄpHŌ¼ĪŖ3£»
¢ó.¼ÓČČ£¬²śÉś“óĮæ×ŲÉ«³Įµķ£¬¾²ÖĆ£¬ÉĻ²ćČÜŅŗ³Ź×ĻŗģÉ«£»
¢ō.¼ÓČėMnSO4ÖĮ×ĻŗģÉ«ĻūŹ§£¬¹żĀĖ£»
¢õ.ÅØĖõ”¢½į¾§”¢·ÖĄė£¬µĆµ½²śĘ·”£
£Ø1£©H2SO4ČܽāAl2O3µÄĄė×Ó·½³ĢŹ½ŹĒ____________________________________”£
£Ø2£©½«MnO4-Ńõ»ÆFe2£«µÄĄė×Ó·½³ĢŹ½²¹³äĶźÕū£ŗ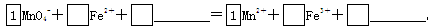
£Ø3£©ŅŃÖŖ£ŗ
Éś³ÉĒāŃõ»ÆĪļ³ĮµķµÄpH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| æŖŹ¼³ĮµķŹ± | 3.4 | 6.3 | 1.5 |
| ĶźČ«³ĮµķŹ± | 4.7 | 8.3 | 2.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ČēĶ¼ŹµŃé×°ÖĆÓĆÓŚŃé֤ijŠ©ĪļÖŹµÄŠŌÖŹ”£ŌŚŹŌ¹ÜA֊װČė×ćĮæµÄ¹ĢĢåNaHCO3,DĪŖ¹Ģ¶ØĪĆĻćµÄÓ²Ö½Ę¬”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ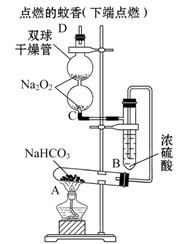
£Ø1£©øĆŹµŃéµÄŹµŃéÄæµÄŹĒ__________________________________________”£
£Ø2£©ŌŚAŹŌ¹ÜÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______________________________”£
£Ø3£©B×°ÖƵÄ×÷ÓĆŹĒ_________________________________________________”£
£Ø4£©ŌŚĖ«ĒņøÉŌļ¹ÜÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________”£
£Ø5£©ŹµŃ鏱¹Ū²ģµ½µÄŹµŃéĻÖĻóŹĒ_______________________________________”£
ÉĻŹöŹµŃéĻÖĻóĖµĆ÷___________________________________________________”£
£Ø6£©Čō½«øÉŌļ¹ÜÄŚµÄNa2O2»»³ÉNa2O£¬ŌņŹµŃ鏱¹Ū²ģµ½µÄŹµŃéĻÖĻóŹĒ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijŠĖȤŠ”×éĪŖ²ā¶Øij¹¤Ņµ“æ¼ī£Ø¼ŁÉč½öŗ¬NaHCO3ŌÓÖŹ£©ÖŠNa2CO3µÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖĻĀĮŠĖÄÖÖŹµŃé·½°ø£ŗ
·½°øŅ»£ŗ“æ¼īѳʷ ²ā¶ØŹ£Óą¹ĢĢåÖŹĮæ
²ā¶ØŹ£Óą¹ĢĢåÖŹĮæ
£Ø1£©³ĘČ”mgѳʷ·ÅČėŪįŪöÖŠ³ä·Ö¼ÓČČ”£ŪįŪöÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
£Ø2£©½«ŪįŪöÖĆÓŚøÉŌļĘ÷ÖŠĄäČ“ŗó³ĘĮ攣ŹµŃ鏱ŠčŅŖÖŲø“”°¼ÓČČ”¢ĄäČ“”¢³ĘĮæ”±²Ł×÷¶ą“Ī£¬ĘäÄæµÄŹĒ£ŗ ”£
·½°ø¶ž£ŗ³ĘČ”mgѳʷÅä³É250mLČÜŅŗ£¬“ÓÖŠČ”25mLČÜŅŗĻČÓĆ·ÓĢŖ×÷ÖøŹ¾¼ĮÓĆ0.1mol/LHClČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄ0.1mol/LHClČÜŅŗV1mL£¬¼ĢŠųÓĆ¼×»ł³Č×÷ÖøŹ¾¼ĮµĪ¶ØÖĮÖÕµć£¬ĻūŗÄ0.1mol/LHClČÜŅŗV2mL”£ŌņøĆѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ£ŗ ”£
·½°øČż£ŗ“æ¼īѳʷČÜŅŗ ²ā¶Ø³ĮµķÖŹĮæ
²ā¶Ø³ĮµķÖŹĮæ
£Ø1£©³ĘČ”m1gѳʷ£¬ÖĆÓŚŠ”ÉÕ±ÖŠ£¬¼ÓĖ®ČܽāŗóµĪ¼Ó×ćĮæĀČ»ÆøĘČÜŅŗ”£½«·“Ó¦»ģŗĶĪļ¹żĀĖŗóµÄĻĀŅ»²½²Ł×÷ŹĒ£ŗ £¬³Įµķ¾øÉŌļŗó³ĘĮæĪŖm2g”£
£Ø2£©Čē¹ūÓĆĒāŃõ»ÆøĘČÜŅŗ“śĢęĀČ»ÆøĘČÜŅŗ×÷³Įµķ¼Į£¬ŌŚĘäĖū²Ł×÷ÕżČ·µÄĒéæöĻĀ£¬_______²ā¶Øѳʷ֊µÄNa2CO3µÄÖŹĮæ·ÖŹż”££ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©
·½°øĖÄ£ŗ“æ¼īѳʷ ²ā¶ØÉś³É¶žŃõ»ÆĢ¼µÄÖŹĮæ
²ā¶ØÉś³É¶žŃõ»ÆĢ¼µÄÖŹĮæ
£Ø1£©±¾ŹµŃé²ÉÓĆČēĶ¼×°ÖĆ£¬CÖŠŹ¢·ÅµÄĪļÖŹŹĒ ”£
£Ø2£©·“Ó¦Ē°ŗó¶¼ŅŖĶØČėN2£¬·“Ó¦ŗóĶØČėN2µÄÄæµÄŹĒ£ŗ 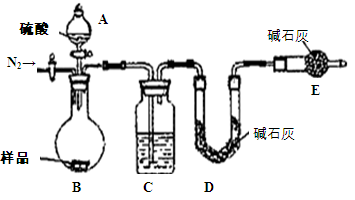
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com