
����Ŀ��(1)�������˻����ƻ�潫�й���ͳ�Ļ������˾����Լ��ִ��߿Ƽ���Ϊһ�塣��������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϡ��Իش��������⣺
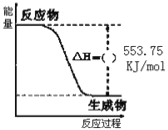
����ͼ��һ����������ȫȼ������CO2��1molH2O(l)�����е������仯ͼ������ͼ�е�������������+�������� ___��
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��___��
�۶�����(CH3OCH3)��һ������ȼ�ϣ�Ӧ��ǰ��������1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ��������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ���������������У�����Ͷ����ѵ����ʵ���֮��Ϊ___��
(2)��˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵�����ЧӦ��ͬ�������ø�˹���ɻش��������⣺
����֪��H2O(g)�TH2O(l) ��H1=Q1kJ/mol
C2H5OH(g)�TC2H5OH(l) ��H2=Q2kJ/mol
C2H5OH(g)+3O2(g)�T2CO2(g)+3H2O(g) ��H3=Q3kJ/mol
��ʹ23gҺ̬��ˮ�ƾ���ȫȼ�գ����ָ������£������������зų�������Ϊ___kJ��
��̼(s)��������Ӧ�����ʱ������COͬʱ����������CO2�������ͨ��ʵ��ֱ�Ӳ�÷�Ӧ��C(s)+![]() O2(g)�TCO(g)����H.�������ʵ�顢���ø�˹���ɼ�����÷�Ӧ����H������ʱ��Ҫ��õ�ʵ��������___��
O2(g)�TCO(g)����H.�������ʵ�顢���ø�˹���ɼ�����÷�Ӧ����H������ʱ��Ҫ��õ�ʵ��������___��
���𰸡� C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol 1:3 1.5Q10.5Q2+0.5Q3 ̼��һ����̼�ı�ȼ����
��������
(1)����ͼ��֪����Ӧ����������������������������
����ͼ���֪��������ȫȼ������1molˮ���ʱ���H=553.75kJ/mol��
�������Ȼ�ѧ����ʽ��ϻ���������ʵ����ͷ�����ʽ����õ������Ѻͱ������ʵ���֮�ȣ�
(2)�����ݸ�˹���ɼ���ɵã�
�����ʵ�顢���ø�˹���ɼ���C(s)+ ![]() O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ���
O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ���
(1)����ͼ��֪����Ӧ�����������������������������÷�ӦΪ��Ӧ���ȣ���HΪ�������ʴ�Ϊ����
����ͼ���֪��������ȫȼ������1molˮ���ʱ���H=553.75kJ/mol����Ӧ���Ȼ�ѧ����ʽΪ��C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol���ʴ�Ϊ��C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol��
��1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ��������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ��������1mol��������ж��������ʵ���x���������ʵ���Ϊ1x�����Ȼ�ѧ����ʽ��֪����ȼ�շ���2215 (1x) kJ��������ɵù�ϵʽ16451455x=2215 (1x)�����x=0.75�����ϱ������ʵ���Ϊ0.25mol����������б���Ͷ��������ʵ���֮��=0.25:0.75=1:3���ʴ�Ϊ��1:3��
(2)�ٽ���֪�Ȼ�ѧ����ʽ���α��Ϊ�١��ڡ��ۣ��ɸ�˹���ɿ�֪������+����3���Ȼ�ѧ����ʽC2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ������H1=(3Q1Q2+Q3)kJ/mol����ʹ23gҺ̬��ˮ�ƾ����ʵ���Ϊ0.5mol����ȫȼ�գ����ָ������£������������зų�������Ϊ(1.5Q10.5Q2+0.5Q3)kJ���ʴ�Ϊ��1.5Q10.5Q2+0.5Q3��
�����ʵ�顢���ø�˹���ɼ���C(s)+ ![]() O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ����ʴ�Ϊ��̼��һ����̼�ı�ȼ���ȡ�
O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ����ʴ�Ϊ��̼��һ����̼�ı�ȼ���ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���У��д������( )
A.Na2CO3��Һ����ʯ��ˮ��Ӧ����NaHCO3��Һ������ʯ��ˮ��Ӧ
B.����ͬ�¶��£�NaHCO3���ܽ�ȱ�Na2CO3С
C.Na2CO3���ȶ�����NaHCO3����ʱ���ֽ�
D.�������ʵ�����NaHCO3��ĩ��Na2CO3��ĩͬʱ�ֱ�����������ͬŨ�ȵ�ϡ�����У�ǰ�ߵķ�Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ��4CO(g)+2NO2(g)=4CO2(g)+N2(g����H=-1200KJ/mol���¶Ȳ�ͬ(T2>T1������������ͬʱ������ͼ����ȷ����

A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£����ܱ������м���һ������C(s)��H2O(g)�������淴ӦC(s) + H2O(g)![]() CO(g) + H2(g)����Ӧ�ﵽƽ���C(s)��H2O(g)ת����Ϊ2:1��ϵ��������˵����ȷ����
CO(g) + H2(g)����Ӧ�ﵽƽ���C(s)��H2O(g)ת����Ϊ2:1��ϵ��������˵����ȷ����
A.��ʼ�����C(s)��H2O(g)���ʵ���Ϊ2:1
B.������ѹǿ��H2O(g)ת���ʲ��仯
C.�ﵽƽ���������ƽ��ʽ������Ϊ16
D.��ʼ��Ӧ��������ƽ��ʽ��һֱ��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����10 L�ܱ������У�1 mol A��3 mol B��һ�������·�Ӧ�� A(g)��xB(g)![]() 2C(g)��2 min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
2C(g)��2 min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
A. ƽ��ʱ�����ʵ���֮��n(A)��n(B)��n(C)��2��11��4
B. xֵ����3
C. A��ת����Ϊ20%
D. B��ƽ����Ӧ����Ϊ0.4 mol/(L��min)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.1 mol��L��1 CH3COOH��Һ�д������µ���ƽ�⣺ CH3COOH ![]() CH3COO����H+�����ڸ�ƽ�⣬����������ȷ���ǣ� ��
CH3COO����H+�����ڸ�ƽ�⣬����������ȷ���ǣ� ��
A. ����ˮʱ��ƽ�����淴Ӧ�����ƶ�
B. ��������NaOH���壬ƽ��������Ӧ�����ƶ�
C. ��������0.1 mol��L��1 HCl��Һ����Һ��c(H+)��С
D. ��������CH3COONa���壬ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ԭ�������ĵ��������ֶ�����Ԫ�أ�����ĸx��y��z������ʾ����ԭ�Ӱ뾶��Դ�С��������ۻ�����۵ı仯����ͼ��ʾ��
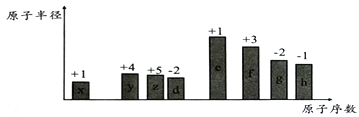
��ش��������⣺
��1��f�����ڱ��е�λ��Ϊ______________��x�γɵ������ӵĽṹʾ��ͼΪ_____________________��
��2���Ƚ�d��e�������ӵİ뾶��С��__________________���ѧʽ����ͬ�����Ƚ�g��h������������Ӧˮ���������ǿ����_______________________��
��3��x��y��z��d����Ԫ�����γɶ��ֻ����
�����γ����ӻ��������һ��x��y��z��d����ԭ�ӵĸ�����Ϊ5��2��1 ��4���仯ѧʽΪ__________________________��
�����γɹ��ۻ����д������һ�ֵĽṹ��ʽ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״���
��1����֪����H2(g)��1/2O2(g)![]() H2O(l) ��H1����285.8 kJ/mol��
H2O(l) ��H1����285.8 kJ/mol��
��CO (g)��1/2O2 (g)![]() CO2 (g) ��H2=��283kJ/mol
CO2 (g) ��H2=��283kJ/mol
��CH3OH(g)��3/2O2(g)![]() CO2(g)��2H2O(l) ��H3����764.6 kJ/mol
CO2(g)��2H2O(l) ��H3����764.6 kJ/mol
��ҵ�Ʊ��״��Ŀ��淴Ӧ�Ȼ�ѧ����ʽΪ_______________________________��
��2�����º��������£�������������˵��������Ӧ�Ѵ�ƽ��״̬����__________��
A����λʱ��������n mol CO��ͬʱ����2n mol H2 B����(H2)����2��(CH3OH)��
C��������������ܶȱ��ֲ��� D�������������ѹǿ���ֲ���
��3��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɼ״��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1 molCO�� 2 molH2��������ʴ�����������Ժ��Բ��ƣ�����250��C��ʼ��Ӧ��CO���ʵ�����ʱ��仯���£�
��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 |
n��CO��/mol | 1.00 | 0.79 | 0.63 | 0.54 | 0.50 | 0.50 |
��ӷ�Ӧ��ʼ��20minʱ��������H2��=________�����¶���ƽ�ⳣ��K��_______��
��4�������������250 ����ʼ��Ӧ��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
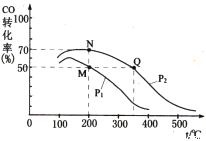
��M��N��Q�����ƽ�ⳣ��KM��KN��KQ�Ĵ�С��ϵΪ___________________________��
����M�㵽N��ı�����������_________��
A�������¶� B������ѹǿ
C�����ø��õĴ��� D��ͨ������CO
��5��25��ʱ��ϡ����Ϊ�������Һ�Ƴɼ״�ȼ�ϵ�أ����ĵ缫����ʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮMgBr2������������ij��ȤС��ͬѧ����þм��Һ��Ϊԭ���Ʊ���ˮMgBr2�����װ����ͼ��ʾ����֪��Mg��Br2��Ӧ���ҷ��ȣ�MgBr2����ǿ��ˮ�ԡ�����˵����ȷ���ǣ� ��

A.����������ˮ�������ڷ������
B.ʵ���п����ø���Ŀ�����������N2
C.Ϊ��ֹ��Ӧ���ھ��ң�������װ��C����װ��B
D.װ����ˮCaCl2����A�����������ջӷ�������������ֹ��Ⱦ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com