
 ��
�� ���������𰸲��ո��֣�
���������𰸲��ո��֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
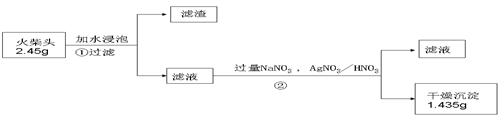
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
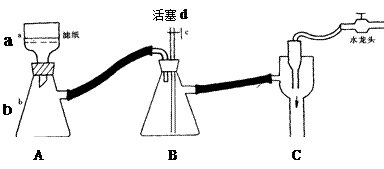
 �ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�
�ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�
 ____________________��
____________________�� �飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������
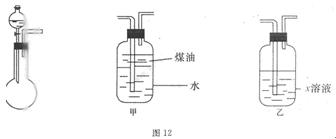
�飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������ ����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������
����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������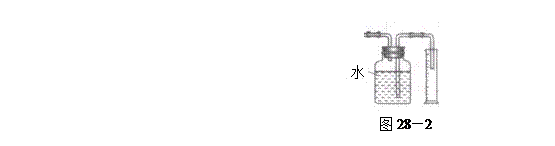
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
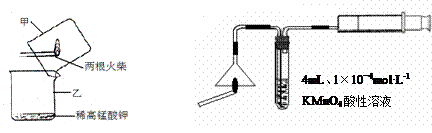
 ��ijѧϰС�����ô�ԭ����
��ijѧϰС�����ô�ԭ���� ����ͼ��ʾװ����ȡ������̽�������ʡ�
����ͼ��ʾװ����ȡ������̽�������ʡ�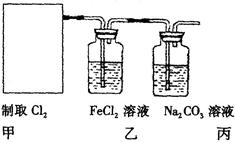
|

 ����װ����FeCl2��ҺCl2��Ӧ�����ӷ���ʽ����������������֤��FeCl2��Cl2������������ԭ��Ӧ��ʵ�鷽���ǣ���
����װ����FeCl2��ҺCl2��Ӧ�����ӷ���ʽ����������������֤��FeCl2��Cl2������������ԭ��Ӧ��ʵ�鷽���ǣ��� ���Լ������ơ���������֣�����������������������
���Լ������ơ���������֣�����������������������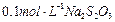 ��Һ��
��Һ�� ��������20mLNa2S2O3��Һ����Ư����Ca(ClO)2����������Ϊ��������������������
��������20mLNa2S2O3��Һ����Ư����Ca(ClO)2����������Ϊ���������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 n��ֵ�����������̽���ʵ�飺
n��ֵ�����������̽���ʵ�飺
�鿴�𰸺ͽ���>>
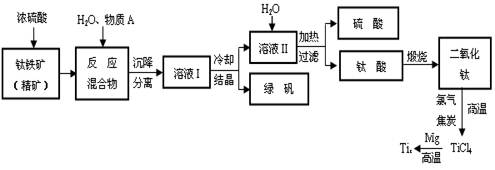
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
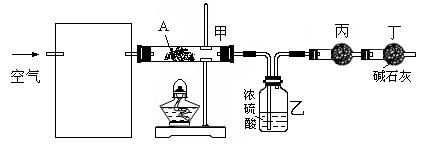
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
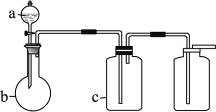
| ��� | ���� | a | b | c |
| A | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
| B | CO2 | ���� | ̼��� | ����NaHCO3 |
| C | NO | ϡHNO3 | ͭм | H2O |
| D | NO2 | ŨHNO3 | ͭм | NaOH��Һ |
�鿴�𰸺ͽ���>>
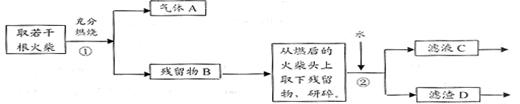
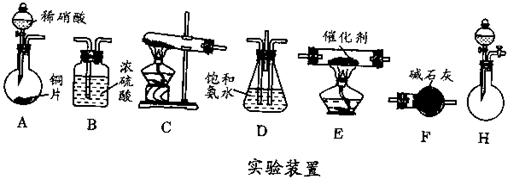
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 _____________��
_____________��
| �Լ������� | �����Լ�/g | NH3���/mL |
| a | 12.0g Ca(OH)2(����) 10.8g NH4Cl | 2688 |
| b | 12.0g Ca (OH)2(����) 10.8g(NH4)2SO4 (OH)2(����) 10.8g(NH4)2SO4 | 2728 |
| c | 12.0g NaOH(����) 10.8g NH4Cl | 3136 |
| d | 12.0g NaOH(����) 10.8g (NH4)2SO4 | 3118 |
| e | 12.0g CaO(����) 10.8g NH4Cl | 3506 |
| f | 12.0g CaO(����) 10.8g (NH4)2SO4 | 3584 |
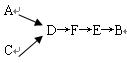
 ____________��
____________���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com