
���� ��1����Ӧ����4mol N-H�����ѣ�����1.5molN2�����ݽṹʽN��N�жϣ����Ӽ京������ķе�ϸߣ�
��2�����ݷ����Цļ��µ��Ӷ����ж��ӻ����ͺͷ��ӵĿռ乹�ͣ�
��3�����Ͱ�����640��ɷ����û���Ӧ���ɵ����͵����������þ�̯��ȷ���������Ļ�ѧʽ�������¶ȡ���Ӧ���������д����Ӧ����ʽ��
��4��a�����ݵ����еĹ��ۼ�������
b�����þ�̯��ȷ���侧���е�ԭ������
c���ǽ�����Խǿ����һ������Խ��ͬ���ڴ����ҵ�һ����������IIA���IIIA����VA�����VIA������
d�����Ӱ뾶ԽС��������Խ��
��5������ͼ��֪̼̼�䡢̼����Ϊ���ۼ���������Ϊ��λ��������������������̼ԭ�ӳɼ������жϣ�
��� �⣺��1��1mol���������к���2mol�м������÷�Ӧ����4mol-H�����ѣ�����1mol�²μӷ�Ӧ������1.5mol�����������γɵĦм���1.5mol��2=3mol��N2H4���Ӽ���γ��������������Ӽ䲻���γ���������Ӽ京������ķе�ϸߣ�����N2H4�е㣨113.5�棩������е㣨-88.6�棩�ߵö࣬
�ʴ�Ϊ��3��N2H4�����γɷ��Ӽ������
��2��NF3�к���3���ļ����ҹµ��Ӷ���Ϊ$\frac{5-3}{2}$=1����ӦΪsp3�ӻ����ռ乹��Ϊ�����Σ�
�ʴ�Ϊ�������Σ�
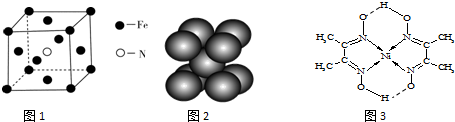
��3���þ�������ԭ�Ӹ���=8��$\frac{1}{8}$����ԭ�Ӹ�����1�����Ե������Ļ�ѧʽ��Fe4N�����Ͱ�����640��ɷ����û���Ӧ���ɵ����͵����������Ը÷�Ӧ����ʽΪ��8Fe+2NH3$\frac{\underline{\;640��\;}}{\;}$2Fe4N+3H2��
�ʴ�Ϊ��8Fe+2NH3$\frac{\underline{\;640��\;}}{\;}$2Fe4N+3H2��
��4��a�������д���N��N��N��N�ļ��ܴܺ������ѣ�����N2���ȶ�����a����
b���ƾ�����Naռ��8����������ģ����ݾ�̯��ȷ���侧���е�ԭ����Ϊ��8��$\frac{1}{8}$+1=2����b����
c���ǽ�����Խǿ����һ������Խ��ͬ���ڴ����ҵ�һ����������IIA���IIIA����VA�����VIA���������Ե�һ�����ܣ�N��O��P��S����c��ȷ��
d�����Ӱ뾶ԽС��������Խ�뾶��Na+��K+�����ܣ�NaN3��KN3����d����
�ʴ�Ϊ��c��
��5������ͼ��֪̼̼���γɷǼ��Թ��ۼ���̼����Ϊ���Թ��ۼ���������Ϊ��λ����������γ����������ͼ��֪��̼̼���γɵ�����Ϊsp3�ӻ����е�̼̼���γ�˫����Ϊsp2�ӻ���
�ʴ�Ϊ��abcd��sp3��sp2��
���� �����ۺ��Խ�ǿ���漰��ѧ�����ӻ��������������ȣ���Ŀ�Ѷ��еȣ�ע�������ӻ������Ƶ����ӹ��ͣ�Ϊ�״��㣬�����ڿ���ѧ������ѧ֪ʶ���ۺ�Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ �� �� �� �� �� �� | ʵ �� �� �� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ��һ������SO${\;}_{4}^{2-}$ |
| B | ��ij��Һ�м������ᣬ�ܲ���ʹ����ʯ��ˮ����ǵ����� | ����Һ��һ����CO32- |
| C | ��ij��Һ�м�������NaOHϡ��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ����ɫ | ����Һ��һ������NH4+ |
| D | �ø���ྻ�IJ�˿պȡij��Һ���ھƾ��ƻ��������գ�����ɫ�ܲ����۲쵽�������ɫ | ����Һһ�����м�Ԫ�أ����ܺ�����Ԫ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ����Уʵ���һ�ѧ�Լ�Ũ�����ǩ�ϵIJ������ݣ�����Ҫ480mL 1mol•L-1��ϡ���ᣮ�ø�Ũ���������ˮ���ƣ��ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ��ڲ����������ձ�������Ͳ��
��ͼ����Уʵ���һ�ѧ�Լ�Ũ�����ǩ�ϵIJ������ݣ�����Ҫ480mL 1mol•L-1��ϡ���ᣮ�ø�Ũ���������ˮ���ƣ��ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ��ڲ����������ձ�������Ͳ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʯī�Ʊ����ʯ�����ȷ�Ӧ��������ʱ��ʯī�������Ƚ��ʯ�ĵ� | |
| B�� | ��ʯī�Ʊ����ʯ�����ȷ�Ӧ��������ʱ��ʯī�������Ƚ��ʯ�ĸ� | |
| C�� | ��ʯī�Ʊ����ʯ�Ƿ��ȷ�Ӧ��������ʱ��ʯī�������Ƚ��ʯ�ĵ� | |
| D�� | ��ʯī�Ʊ����ʯ�Ƿ��ȷ�Ӧ��������ʱ��ʯī�������Ƚ��ʯ�ĸ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ࡢ��֬�������ʶ�����C��H��O����Ԫ����ɵ� | |
| B�� | ���ࡢ��֬�������ʶ�����Ȼ�ĸ߷��ӻ����� | |
| C�� | ֲ������ʹ��ˮ��ɫ���⻯�����⣩���Ա�Ϊ֬�� | |
| D�� | ���ۡ���������Һ���ȡ��ȶ��������������ЧӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2.0mol/L | B�� | 1.0mol/L | C�� | 0.5mol/L | D�� | 0.2mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com