
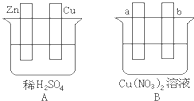
 ���õ��߽�A��B��װ�õ��ĸ��缫�������ӣ�ʹa������ͭ���ش��й����⣮
���õ��߽�A��B��װ�õ��ĸ��缫�������ӣ�ʹa������ͭ���ش��й����⣮


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡִ����ѧ�߶���ѧ����ĩ�������ƻ�ѧ�Ծ����������� ���ͣ������
��.�õ��߽�A��B��װ�õ��ĸ��缫�������ӣ�ʹa������ͭ���ش��й����⡣
��1�����߽�����A��B����ʱ��Zn�� ��Cu�� ���a����b����
��2������A��Cu�������ĵ缫��ӦΪ ��
��3��Bװ�ý� ����Һ�е�NO3-��_____���ƶ����a����b������
��4����b���۲쵽����ɫ��ζ���ݲ���, ����һ��ʱ���ֹͣ��Ӧ��������ȣ���Һ��pHֵ�� ������ߡ��������͡����䡱��������һ������ ���ѧʽ������Һ�ָܻ����뷴Ӧǰ��ȫһ�¡�����Ӧһ��ʱ������Һ��Cu2+Ũ��û�������½������ܵ�ԭ���ǣ� ��
��.���˵�����û��ϴ�ɾ��������⡣�ñ�Ҫ�����ֺ��йػ�ѧ����ʽ˵������������γɵ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ�߶���ѧ����ĩ�������ƻ�ѧ�Ծ��������棩 ���ͣ������
��.�õ��߽�A��B��װ�õ��ĸ��缫�������ӣ�ʹa������ͭ���ش��й����⡣

��1�����߽�����A��B����ʱ��Zn�� ��Cu�� ���a����b����
��2������A��Cu�������ĵ缫��ӦΪ ��
��3��Bװ�ý� ����Һ�е�NO3-��_____���ƶ����a����b������
��4����b���۲쵽����ɫ��ζ���ݲ���, ����һ��ʱ���ֹͣ��Ӧ��������ȣ���Һ��pHֵ�� ������ߡ��������͡����䡱��������һ������ ���ѧʽ������Һ�ָܻ����뷴Ӧǰ��ȫһ�¡�����Ӧһ��ʱ������Һ��Cu2+Ũ��û�������½������ܵ�ԭ���ǣ� ��
��.���˵�����û��ϴ�ɾ��������⡣�ñ�Ҫ�����ֺ��йػ�ѧ����ʽ˵������������γɵ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ������ִ����ѧ�߶����ϣ���ĩ��ѧ�Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com